Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development
- PMID: 22791303
- PMCID: PMC3440208
- DOI: 10.1104/pp.112.197954
Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development
Abstract
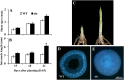
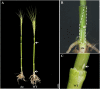
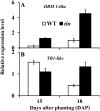
Tillering (branching) is a major yield component and, therefore, a target for improving the yield of crops. However, tillering is regulated by complex interactions of endogenous and environmental signals, and the knowledge required to achieve optimal tiller number through genetic and agronomic means is still lacking. Regulatory mechanisms may be revealed through physiological and molecular characterization of naturally occurring and induced tillering mutants in the major crops. Here we characterize a reduced tillering (tin, for tiller inhibition) mutant of wheat (Triticum aestivum). The reduced tillering in tin is due to early cessation of tiller bud outgrowth during the transition of the shoot apex from the vegetative to the reproductive stage. There was no observed difference in the development of the main stem shoot apex between tin and the wild type. However, tin initiated internode development earlier and, unlike the wild type, the basal internodes in tin were solid rather than hollow. We hypothesize that tin represents a novel type of reduced tillering mutant associated with precocious internode elongation that diverts sucrose (Suc) away from developing tillers. Consistent with this hypothesis, we have observed upregulation of a gene induced by Suc starvation, downregulation of a Suc-inducible gene, and a reduced Suc content in dormant tin buds. The increased expression of the wheat Dormancy-associated (DRM1-like) and Teosinte Branched1 (TB1-like) genes and the reduced expression of cell cycle genes also indicate bud dormancy in tin. These results highlight the significance of Suc in shoot branching and the possibility of optimizing tillering by manipulating the timing of internode elongation.
Figures







Similar articles
-
Overexpression of the maize Teosinte Branched1 gene in wheat suppresses tiller development.Plant Cell Rep. 2008 Jul;27(7):1217-25. doi: 10.1007/s00299-008-0543-8. Epub 2008 Apr 5. Plant Cell Rep. 2008. PMID: 18392625
-
Optimizing tiller production and survival for grain yield improvement in a bread wheat × spelt mapping population.Ann Bot. 2016 Jan;117(1):51-66. doi: 10.1093/aob/mcv147. Epub 2015 Sep 30. Ann Bot. 2016. PMID: 26424785 Free PMC article.
-
Physiological perspectives of reduced tillering and stunting in the tiller inhibition (tin) mutant of wheat.Funct Plant Biol. 2013 Oct;40(10):977-985. doi: 10.1071/FP13034. Funct Plant Biol. 2013. PMID: 32481166
-
Unlocking the Multifaceted Mechanisms of Bud Outgrowth: Advances in Understanding Shoot Branching.Plants (Basel). 2023 Oct 20;12(20):3628. doi: 10.3390/plants12203628. Plants (Basel). 2023. PMID: 37896091 Free PMC article. Review.
-
Tillering and panicle branching genes in rice.Gene. 2014 Mar 1;537(1):1-5. doi: 10.1016/j.gene.2013.11.058. Epub 2013 Dec 15. Gene. 2014. PMID: 24345551 Review.
Cited by
-
Strigolactone and abscisic acid synthesis and signaling pathways are enhanced in the wheat oligo-tillering mutant ot1.Mol Breed. 2024 Feb 2;44(2):12. doi: 10.1007/s11032-024-01450-3. eCollection 2024 Feb. Mol Breed. 2024. PMID: 38313680 Free PMC article.
-
Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida.J Exp Bot. 2015 May;66(9):2569-82. doi: 10.1093/jxb/erv047. Epub 2015 Apr 13. J Exp Bot. 2015. PMID: 25873679 Free PMC article.
-
The LONELY GUY gene family: from mosses to wheat, the key to the formation of active cytokinins in plants.Plant Biotechnol J. 2022 Apr;20(4):625-645. doi: 10.1111/pbi.13783. Epub 2022 Mar 1. Plant Biotechnol J. 2022. PMID: 35108444 Free PMC article. Review.
-
Major genes determining yield-related traits in wheat and barley.Theor Appl Genet. 2017 Jun;130(6):1081-1098. doi: 10.1007/s00122-017-2880-x. Epub 2017 Mar 17. Theor Appl Genet. 2017. PMID: 28314933 Free PMC article. Review.
-
Shade signals activate distinct molecular mechanisms that induce dormancy and inhibit flowering in vegetative axillary buds of sorghum.Plant Direct. 2024 Aug 19;8(8):e626. doi: 10.1002/pld3.626. eCollection 2024 Sep. Plant Direct. 2024. PMID: 39166257 Free PMC article.
References
-
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 - PubMed
-
- Atsmon D, Bush MG, Evans LT. (1986) Stunting in gigas wheat as influenced by temperature and daylength. Aust J Plant Physiol 13: 381–389
-
- Atsmon D, Jacobs E. (1977) Newly bred gigas form of bread wheat (Triticum aestivum L): morphological features and thermo-photoperiodic responses. Crop Sci 17: 31–35
-
- Ballard LAT, Wildman SG. (1964) Induction of mitosis by sucrose in excised and attached dormant buds of sunflower (Helianthus annuus L). Aust J Biol Sci 17: 36–43
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

