Impact of nitric oxide-mediated vasodilation on outer medullary NaCl transport and oxygenation
- PMID: 22791340
- PMCID: PMC3469683
- DOI: 10.1152/ajprenal.00055.2012
Impact of nitric oxide-mediated vasodilation on outer medullary NaCl transport and oxygenation
Abstract
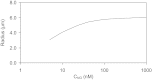
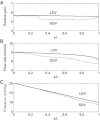
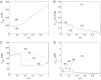
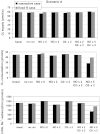
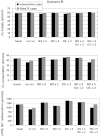
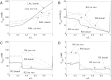
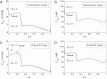
The present study aimed to elucidate the reciprocal interactions between oxygen (O(2)), nitric oxide (NO), and superoxide (O(2)(-)) and their effects on vascular and tubular function in the outer medulla. We expanded our region-based model of transport in the rat outer medulla (Edwards A, Layton AT. Am J Physiol Renal Physiol 301: F979-F996, 2011) to incorporate the effects of NO on descending vasa recta (DVR) diameter and blood flow. Our model predicts that the segregation of long DVR in the center of vascular bundles, away from tubular segments, gives rise to large radial NO concentration gradients that in turn result in differential regulation of vasoactivity in short and long DVR. The relative isolation of long DVR shields them from changes in the rate of NaCl reabsorption, and hence from changes in O(2) requirements, by medullary thick ascending limbs (mTALs), thereby preserving O(2) delivery to the inner medulla. The model also predicts that O(2)(-) can sufficiently decrease the bioavailability of NO in the interbundle region to affect the diameter of short DVR, suggesting that the experimentally observed effects of O(2)(-) on medullary blood flow may be at least partly mediated by NO. In addition, our results indicate that the tubulovascular cross talk of NO, that is, the diffusion of NO produced by mTAL epithelia toward adjacent DVR, helps to maintain blood flow and O(2) supply to the interbundle region even under basal conditions. NO also acts to preserve local O(2) availability by inhibiting the rate of active Na(+) transport, thereby reducing the O(2) requirements of mTALs. The dual regulation by NO of oxygen supply and demand is predicted to significantly attenuate the hypoxic effects of angiotensin II.
Figures


Similar articles
-
Nitric oxide and superoxide transport in a cross section of the rat outer medulla. II. Reciprocal interactions and tubulovascular cross talk.Am J Physiol Renal Physiol. 2010 Sep;299(3):F634-47. doi: 10.1152/ajprenal.00681.2009. Epub 2010 Jun 2. Am J Physiol Renal Physiol. 2010. PMID: 20519375 Free PMC article.
-
Modulation of outer medullary NaCl transport and oxygenation by nitric oxide and superoxide.Am J Physiol Renal Physiol. 2011 Nov;301(5):F979-96. doi: 10.1152/ajprenal.00096.2011. Epub 2011 Aug 17. Am J Physiol Renal Physiol. 2011. PMID: 21849492 Free PMC article.
-
Nitric oxide and superoxide transport in a cross section of the rat outer medulla. I. Effects of low medullary oxygen tension.Am J Physiol Renal Physiol. 2010 Sep;299(3):F616-33. doi: 10.1152/ajprenal.00680.2009. Epub 2010 Jun 9. Am J Physiol Renal Physiol. 2010. PMID: 20534869 Free PMC article.
-
Molecular mechanisms and therapeutic strategies of chronic renal injury: physiological role of angiotensin II-induced oxidative stress in renal medulla.J Pharmacol Sci. 2006 Jan;100(1):2-8. doi: 10.1254/jphs.fmj05003x2. Epub 2006 Jan 11. J Pharmacol Sci. 2006. PMID: 16404134 Review.
-
Nitric oxide and superoxide in the renal medulla: a delicate balancing act.Curr Opin Nephrol Hypertens. 2005 Jan;14(1):9-15. doi: 10.1097/00041552-200501000-00003. Curr Opin Nephrol Hypertens. 2005. PMID: 15586010 Review.
Cited by
-
Oxygen transport in a cross section of the rat inner medulla: impact of heterogeneous distribution of nephrons and vessels.Math Biosci. 2014 Dec;258:68-76. doi: 10.1016/j.mbs.2014.09.009. Epub 2014 Sep 28. Math Biosci. 2014. PMID: 25260928 Free PMC article.
-
Adaptive responses of rat descending vasa recta to ischemia.Am J Physiol Renal Physiol. 2018 Mar 1;314(3):F373-F380. doi: 10.1152/ajprenal.00062.2017. Epub 2017 Aug 16. Am J Physiol Renal Physiol. 2018. PMID: 28814437 Free PMC article.
-
Renal Medulla in Hypertension.Hypertension. 2024 Dec;81(12):2383-2394. doi: 10.1161/HYPERTENSIONAHA.124.21711. Epub 2024 Sep 30. Hypertension. 2024. PMID: 39344517 Review.
-
Renal medullary and urinary oxygen tension during cardiopulmonary bypass in the rat.Math Med Biol. 2017 Sep 1;34(3):313-333. doi: 10.1093/imammb/dqw010. Math Med Biol. 2017. PMID: 27281792 Free PMC article.
References
-
- Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985 - PubMed
-
- Brezis M, Rosen S, Silva P, Epstein F. Renal ischemia: a new perspective. Kidney Int 26: 375–383, 1984 - PubMed
-
- Canton AD, Stanziale R, Corradi A, Andreucci VE, Migone VE. Effects of acute ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int 12: 403–411, 1977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

