Dynamin2 S-nitrosylation regulates adenovirus type 5 infection of epithelial cells
- PMID: 22791607
- PMCID: PMC3541785
- DOI: 10.1099/vir.0.042713-0
Dynamin2 S-nitrosylation regulates adenovirus type 5 infection of epithelial cells
Abstract
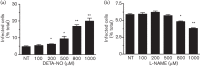
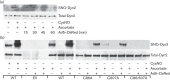
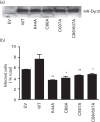
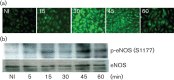
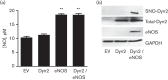
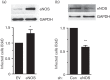
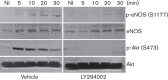
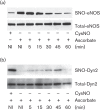
Dynamin2 is a large GTPase that regulates vesicle trafficking, and the GTPase activity of dynamin2 is required for the multistep process of adenovirus infection. Activity of dynamin2 may be regulated by post-translational phosphorylation and S-nitrosylation modifications. In this study, we demonstrate a role for dynamin2 S-nitrosylation in adenovirus infection of epithelial cells. We show that adenovirus serotype 5 (Ad5) infection augments production of nitric oxide (NO) in epithelial cells and causes the S-nitrosylation of dynamin2, mainly on cysteine 86 (C86) and 607 (C607) residues. Forced overexpression of dynamin2 bearing C86A and/or C607A mutations decreases Ad5 infection. Diminishing NO synthesis by RNAi-induced knockdown of endogenous endothelial NO synthase (eNOS) expression attenuates virus infection of target cells. Ad5 infection promotes the kinetically dynamic S-nitrosylation of dynamin2 and eNOS: there is a rapid decrease in eNOS S-nitrosylation and a concomitant increase in the dynamin2 S-nitrosylation. These results support the hypothesis that dynamin2 S-nitrosylation following eNOS activation facilitates adenovirus infection of host epithelial cells.
Figures
References
-
- Ahn S., Kim J., Lucaveche C. L., Reedy M. C., Luttrell L. M., Lefkowitz R. J., Daaka Y. (2002). Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem 277, 26642–26651 10.1074/jbc.M201499200 - DOI - PubMed
-
- Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. (2003). Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278, 14841–14849 10.1074/jbc.M211926200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

