Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates
- PMID: 22791718
- PMCID: PMC3436318
- DOI: 10.1074/jbc.M112.362194
Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates
Abstract
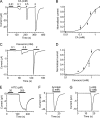
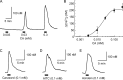
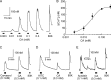
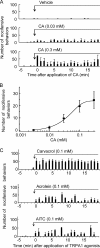
Transient receptor potential ankyrin 1 (TRPA1) and TRP vanilloid 1 (V1) perceive noxious temperatures and chemical stimuli and are involved in pain sensation in mammals. Thus, these two channels provide a model for understanding how different genes with similar biological roles may influence the function of one another during the course of evolution. However, the temperature sensitivity of TRPA1 in ancestral vertebrates and its evolutionary path are unknown as its temperature sensitivities vary among different vertebrate species. To elucidate the functional evolution of TRPA1, TRPA1s of the western clawed (WC) frogs and green anole lizards were characterized. WC frog TRPA1 was activated by heat and noxious chemicals that activate mammalian TRPA1. These stimuli also activated native sensory neurons and elicited nocifensive behaviors in WC frogs. Similar to mammals, TRPA1 was functionally co-expressed with TRPV1, another heat- and chemical-sensitive nociceptive receptor, in native sensory neurons of the WC frog. Green anole TRPA1 was also activated by heat and noxious chemical stimulation. These results suggest that TRPA1 was likely a noxious heat and chemical receptor and co-expressed with TRPV1 in the nociceptive sensory neurons of ancestral vertebrates. Conservation of TRPV1 heat sensitivity throughout vertebrate evolution could have changed functional constraints on TRPA1 and influenced the functional evolution of TRPA1 regarding temperature sensitivity, whereas conserving its noxious chemical sensitivity. In addition, our results also demonstrated that two mammalian TRPA1 inhibitors elicited different effect on the TRPA1s of WC frogs and green anoles, which can be utilized to clarify the structural bases for inhibition of TRPA1.
Figures




Similar articles
-
Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison.Mol Biol Evol. 2014 Mar;31(3):708-22. doi: 10.1093/molbev/msu001. Epub 2014 Jan 7. Mol Biol Evol. 2014. PMID: 24398321
-
Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs.PLoS Genet. 2011 Apr;7(4):e1002041. doi: 10.1371/journal.pgen.1002041. Epub 2011 Apr 7. PLoS Genet. 2011. PMID: 21490957 Free PMC article.
-
Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates.J Biol Chem. 2012 Jan 20;287(4):2388-97. doi: 10.1074/jbc.M111.305698. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130664 Free PMC article.
-
Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates.Temperature (Austin). 2017 Apr 7;4(2):141-152. doi: 10.1080/23328940.2017.1315478. eCollection 2017. Temperature (Austin). 2017. PMID: 28680930 Free PMC article. Review.
-
[Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1].Yakugaku Zasshi. 2010 Mar;130(3):289-94. doi: 10.1248/yakushi.130.289. Yakugaku Zasshi. 2010. PMID: 20190512 Review. Japanese.
Cited by
-
The role of allosteric coupling on thermal activation of thermo-TRP channels.Biophys J. 2013 May 21;104(10):2160-9. doi: 10.1016/j.bpj.2013.03.055. Biophys J. 2013. PMID: 23708356 Free PMC article.
-
Molecular mechanisms of temperature adaptation.J Physiol. 2015 Aug 15;593(16):3483-91. doi: 10.1113/jphysiol.2014.280446. Epub 2015 Jan 5. J Physiol. 2015. PMID: 25433072 Free PMC article. Review.
-
Human and Mouse TRPA1 Are Heat and Cold Sensors Differentially Tuned by Voltage.Cells. 2019 Dec 24;9(1):57. doi: 10.3390/cells9010057. Cells. 2019. PMID: 31878344 Free PMC article.
-
Nociception and hypersensitivity involve distinct neurons and molecular transducers in Drosophila.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2113645119. doi: 10.1073/pnas.2113645119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294287 Free PMC article.
-
Differences in ion channel phenotype and function between humans and animal models.Front Biosci (Landmark Ed). 2018 Jan 1;23(1):43-64. doi: 10.2741/4581. Front Biosci (Landmark Ed). 2018. PMID: 28930537 Free PMC article. Review.
References
-
- Patapoutian A., Peier A. M., Story G. M., Viswanath V. (2003) ThermoTRP channels and beyond. Mechanisms of temperature sensation. Nat. Rev. Neurosci. 4, 529–539 - PubMed
-
- Dhaka A., Viswanath V., Patapoutian A. (2006) TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161 - PubMed
-
- Kwan K. Y., Allchorne A. J., Vollrath M. A., Christensen A. P., Zhang D. S., Woolf C. J., Corey D. P. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 - PubMed
-
- Bandell M., Story G. M., Hwang S. W., Viswanath V., Eid S. R., Petrus M. J., Earley T. J., Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

