Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis
- PMID: 22791749
- PMCID: PMC3459463
- DOI: 10.1093/hmg/dds272
Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis
Abstract
Male patients with Peutz-Jeghers syndrome (PJS) have defective spermatogenesis and are at increased risk of developing Sertoli cell tumors. Mutations in the Liver Kinase B1 (LKB1/STK11) gene are associated with the pathogenesis of PJS and have been identified in non-PJS patients with sporadic testicular cancers. The mechanisms controlled by LKB1 signaling in Sertoli cell functions and testicular biology have not been described. We have conditionally deleted the Lkb1 gene (Lkb1(cko)) in somatic testicular cells to define the molecular mechanisms involved in the development of the testicular phenotype observed in PJS patients. Focal vacuolization in some of the seminiferous tubules was observed in 4-week-old mutant testes but germ cell development appeared to be normal. However, similar to PJS patients, we observed progressive germ cell loss and Sertoli cell only tubules in Lkb1(cko) testes from mice older than 10 weeks, accompanied by defects in Sertoli cell polarity and testicular junctional complexes and decreased activation of the MAP/microtubule affinity regulating and focal adhesion kinases. Suppression of AMP kinase and activation of mammalian target of rapamycin (mTOR) signaling were also observed in Lkb1(cko) testes. Loss of Tsc1 or Tsc2 copies the progressive Lkb1(cko) phenotype, suggesting that dysregulated activation of mTOR contributes to the pathogenesis of the Lkb1(cko) testicular phenotype. Pten(cko) mice had a normal testicular phenotype, which could be explained by the comparative lack of mTOR activation detected. These studies describe the importance of LKB1 signaling in testicular biology and the possible molecular mechanisms driving the pathogenesis of the testicular defects observed in PJS patients.
Figures
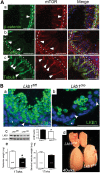
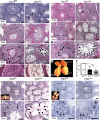

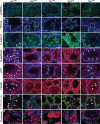
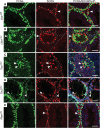
References
-
- Hezel A.F., Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi:10.1038/onc.2008.342. - DOI - PubMed
-
- Jeghers H., Mc K.V., Katz K.H. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N. Engl. J. Med. 1949;241:1031–1036. doi:10.1056/NEJM194912292412601. - DOI - PubMed
-
- Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Hoglund P., et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi:10.1038/34432. - DOI - PubMed
-
- Jenne D.E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Muller O., Back W., Zimmer M. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998;18:38–43. doi:10.1038/ng0198-38. - DOI - PubMed
-
- Shackelford D.B., Shaw R.J. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi:10.1038/nrc2676. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

