Fractionation of social brain circuits in autism spectrum disorders
- PMID: 22791801
- PMCID: PMC3437021
- DOI: 10.1093/brain/aws160
Fractionation of social brain circuits in autism spectrum disorders
Abstract
Autism spectrum disorders are developmental disorders characterized by impairments in social and communication abilities and repetitive behaviours. Converging neuroscientific evidence has suggested that the neuropathology of autism spectrum disorders is widely distributed, involving impaired connectivity throughout the brain. Here, we evaluate the hypothesis that decreased connectivity in high-functioning adolescents with an autism spectrum disorder relative to typically developing adolescents is concentrated within domain-specific circuits that are specialized for social processing. Using a novel whole-brain connectivity approach in functional magnetic resonance imaging, we found that not only are decreases in connectivity most pronounced between regions of the social brain but also they are selective to connections between limbic-related brain regions involved in affective aspects of social processing from other parts of the social brain that support language and sensorimotor processes. This selective pattern was independently obtained for correlations with measures of social symptom severity, implying a fractionation of the social brain in autism spectrum disorders at the level of whole circuits.
Figures
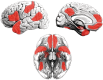
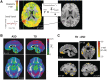
 ; see colour bar). (C) The normally distributed group maps could then be compared using t-tests (P < 0.05, two-tailed, minimum cluster size = 100 contiguous voxels). Data for this and subsequent figures are plotted in standard anatomical coordinates (Talairach-Tournoux).
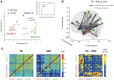
; see colour bar). (C) The normally distributed group maps could then be compared using t-tests (P < 0.05, two-tailed, minimum cluster size = 100 contiguous voxels). Data for this and subsequent figures are plotted in standard anatomical coordinates (Talairach-Tournoux).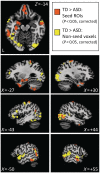
Comment in
-
Autism and intellectual disability.J Neurol. 2013 Mar;260(3):936-9. doi: 10.1007/s00415-013-6861-y. J Neurol. 2013. PMID: 23423245 No abstract available.
References
-
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–78. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. - PubMed
-
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. - PubMed

