Both stromal cell and colonocyte epidermal growth factor receptors control HCT116 colon cancer cell growth in tumor xenografts
- PMID: 22791816
- PMCID: PMC3529558
- DOI: 10.1093/carcin/bgs231
Both stromal cell and colonocyte epidermal growth factor receptors control HCT116 colon cancer cell growth in tumor xenografts
Abstract
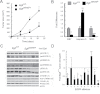
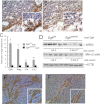
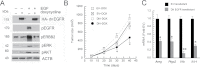
Colon cancer growth requires growth-promoting interactions between malignant colonocytes and stromal cells. Epidermal growth factor receptors (EGFR) are expressed on colonocytes and many stromal cells. Furthermore, EGFR is required for efficient tumorigenesis in experimental colon cancer models. To dissect the cell-specific role of EGFR, we manipulated receptor function on stromal cells and cancer cells. To assess the role of stromal EGFR, HCT116 human colon cancer cells were implanted into immunodeficient mice expressing dominant negative (DN) Egfr(Velvet/+) or Egfr(+/+). To assess the role of cancer cell EGFR, HCT116 transfectants expressing inducible DN-Egfr were implanted into immunodeficient mice. To dissect EGFR signals in vitro, we examined colon cancer cells in monoculture or in cocultures with fibroblasts for EGFR transactivation and prostaglandin synthase 2 (PTGS2) induction. EGFR signals were determined by blotting, immunostaining and real-time PCR. Tumor xenografts in Egfr(Velvet/+) mice were significantly smaller than tumors in Egfr(+/+) mice, with decreased proliferation (Ki67) and increased apoptosis (cleaved caspase-3) in cancer cells and decreased stromal blood vessels. Mouse stromal transforming growth factor alpha (TGFA), amphiregulin (AREG), PTGS2 and Il1b and interleukin-1 receptor 1 (Il1r1) transcripts and cancer cell beta catenin (CTNNB1) and cyclin D1 (CCND1) were significantly lower in tumors obtained from Egfr(Velvet/+) mice. DN-EGFR HCT116 transfectants also formed significantly smaller tumors with reduced mouse Areg, Ptgs2, Il1b and Il1r1 transcripts. Coculture increased Caco-2 phospho-active ERBB (pERBB2), whereas DN-EGFR in Caco-2 cells suppressed fibroblast PTGS2 and prostaglandin E2 (PGE2). In monoculture, interleukin 1 beta (IL1B) transactivated EGFR in HCT116 cells. Stromal cell and colonocyte EGFRs are required for robust EGFR signals and efficient tumor growth, which involve EGFR-interleukin-1 crosstalk.
Conflict of interest statement
Figures



References
-
- Li H., et al. (2007). Tumor microenvironment: the role of the tumor stroma in cancer J Cell Biochem., 101 805–815 - PubMed
-
- Inatomi O., et al. (2006). Regulation of amphiregulin and epiregulin expression in human colonic subepithelial myofibroblasts Int. J. Mol. Med. 18 497–503 - PubMed
-
- Blanchot-Jossic F., et al. (2005). Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells J. Pathol. 207 156–163 - PubMed
-
- Barnard J.A., et al. (1995). Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology Gastroenterology 108 564–580 - PubMed
-
- Inatomi O., et al. (2006). Regulation of amphiregulin and epiregulin expression in human colonic subepithelial myofibroblasts Int. J. Mol. Med. 18 497–503 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

