RTK signaling modulates the Dorsal gradient
- PMID: 22791891
- PMCID: PMC3403108
- DOI: 10.1242/dev.075812
RTK signaling modulates the Dorsal gradient
Abstract
The dorsoventral (DV) axis of the Drosophila embryo is patterned by a nuclear gradient of the Rel family transcription factor, Dorsal (Dl), that activates or represses numerous target genes in a region-specific manner. Here, we demonstrate that signaling by receptor tyrosine kinases (RTK) reduces nuclear levels and transcriptional activity of Dl, both at the poles and in the mid-body of the embryo. These effects depend on wntD, which encodes a Dl antagonist belonging to the Wingless/Wnt family of secreted factors. Specifically, we show that, via relief of Groucho- and Capicua-mediated repression, the Torso and EGFR RTK pathways induce expression of WntD, which in turn limits Dl nuclear localization at the poles and along the DV axis. Furthermore, this RTK-dependent control of Dl is important for restricting expression of its targets in both contexts. Thus, our results reveal a new mechanism of crosstalk, whereby RTK signals modulate the spatial distribution and activity of a developmental morphogen in vivo.
Figures




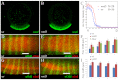

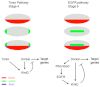
Similar articles
-
Capicua controls Toll/IL-1 signaling targets independently of RTK regulation.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):1807-1812. doi: 10.1073/pnas.1713930115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432195 Free PMC article.
-
Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila.Development. 2012 Mar;139(6):1110-4. doi: 10.1242/dev.076562. Epub 2012 Feb 8. Development. 2012. PMID: 22318229
-
A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling.EMBO J. 2007 Feb 7;26(3):668-77. doi: 10.1038/sj.emboj.7601532. Epub 2007 Jan 25. EMBO J. 2007. PMID: 17255944 Free PMC article.
-
The Capicua repressor--a general sensor of RTK signaling in development and disease.J Cell Sci. 2012 Mar 15;125(Pt 6):1383-91. doi: 10.1242/jcs.092965. J Cell Sci. 2012. PMID: 22526417 Free PMC article. Review.
-
Temporal dynamics, spatial range, and transcriptional interpretation of the Dorsal morphogen gradient.Curr Opin Genet Dev. 2012 Dec;22(6):542-6. doi: 10.1016/j.gde.2012.08.005. Epub 2012 Sep 13. Curr Opin Genet Dev. 2012. PMID: 22981910 Review.
Cited by
-
Epidermal Growth Factor Pathway Signaling in Drosophila Embryogenesis: Tools for Understanding Cancer.Cancers (Basel). 2017 Feb 7;9(2):16. doi: 10.3390/cancers9020016. Cancers (Basel). 2017. PMID: 28178204 Free PMC article. Review.
-
A non-canonical Raf function is required for dorsal-ventral patterning during Drosophila embryogenesis.Sci Rep. 2022 May 10;12(1):7684. doi: 10.1038/s41598-022-11699-3. Sci Rep. 2022. PMID: 35538124 Free PMC article.
-
Transfer of Dorsoventral and Terminal Information from the Ovary to the Embryo by a Common Group of Eggshell Proteins in Drosophila.Genetics. 2017 Apr;205(4):1529-1536. doi: 10.1534/genetics.116.197574. Epub 2017 Feb 7. Genetics. 2017. PMID: 28179368 Free PMC article.
-
Novel interplay between JNK and Egfr signaling in Drosophila dorsal closure.PLoS Genet. 2017 Jun 19;13(6):e1006860. doi: 10.1371/journal.pgen.1006860. eCollection 2017 Jun. PLoS Genet. 2017. PMID: 28628612 Free PMC article.
-
Temporal integration of inductive cues on the way to gastrulation.Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2102691118. doi: 10.1073/pnas.2102691118. Proc Natl Acad Sci U S A. 2021. PMID: 34083443 Free PMC article.
References
-
- Brönner G., Jäckle H. (1991). Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mech. Dev. 35, 205–211 - PubMed
-
- Casanova J. (1991). Interaction between torso and dorsal, two elements of different transduction pathways in the Drosophila embryo. Mech. Dev. 36, 41–45 - PubMed
-
- Chopra V. S., Levine M. (2009). Combinatorial patterning mechanisms in the Drosophila embryo. Brief. Funct. Genomics Proteomics 8, 243–249 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

