Activation of Stat3 in endothelial cells following hypoxia-reoxygenation is mediated by Rac1 and protein Kinase C
- PMID: 22791907
- PMCID: PMC3392026
- DOI: 10.1016/j.bbamcr.2012.02.008
Activation of Stat3 in endothelial cells following hypoxia-reoxygenation is mediated by Rac1 and protein Kinase C
Abstract
Stat3 is an important transcription factor that regulates both proinflammatory and anit-apoptotic pathways in the heart. This study examined the mechanisms of activation of Stat3 in human endothelial cells following hypoxia/reoxygenation (H/R). By expression of constitutively active Rac1 mutant protein, and by RNA silencing of Rac1, we found that Stat3 forms a multiprotein complex with Rac1 and PKC in an H/R-dependent manner, which at least in part, appears to regulate Stat3 S727 phosphorylation. Selective inhibition of PKC with calphostin C produces a marked suppression of Stat3 S727 phosphorylation. The association of Stat3 with Rax1 occurs predominantly at the cell membrane, but also inside the nucleus, and occurs through the binding of the coiled-coil domain of Stat3 to the 54 NH(2)-terminal residues of Rac1. Transfection with a peptide comprising the NH(2)-terminal 17 amino acid residues of Rac1-dependent signaling pathways resulting in physical association between Rac1 and Stat3 and the formation of a novel multiprotein complex with PKC.
Keywords: Stat3; endothelial cells; inflammation; protein kinase C; protein-protein interaction; reactive oxygen species.
Figures
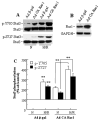







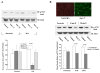
References
-
- Das DK. Redox regulation of cardiomyocyte survival and death. Antioxid Redox Signal. 2001;3:23–37. - PubMed
-
- Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res Cardiol. 2004;99:1–7. - PubMed
-
- Soond SM, Latchman DS, Stephanou A. STAT signalling in the heart and cardioprotection. Expert Rev Mol Med. 2006;8:1–16. - PubMed
-
- Yang XP, Irani K, Mattagajasingh S, Dipaula A, Khanday F, Ozaki M, Fox-Talbot K, Baldwin WM, 3rd, Becker LC. Signal transducer and activator of transcription 3alpha and specificity protein 1 interact to upregulate intercellular adhesion molecule-1 in ischemic-reperfused myocardium and vascular endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1395–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

