RBPJ is a novel target for rhabdomyosarcoma therapy
- PMID: 22792167
- PMCID: PMC3392254
- DOI: 10.1371/journal.pone.0039268
RBPJ is a novel target for rhabdomyosarcoma therapy
Abstract
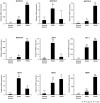
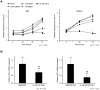
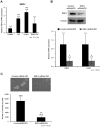
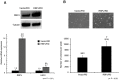
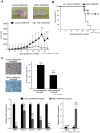
The Notch pathway regulates a broad spectrum of cell fate decisions and differentiation processes during fetal and postnatal development. In addition, the Notch pathway plays an important role in controlling tumorigenesis. However, the role of RBPJ, a transcription factor in the Notch pathway, in the development of tumors is largely unknown. In this study, we focused on the role of RBPJ in the pathogenesis of rhabdomyosarcoma (RMS). Our data showed that Notch pathway genes were upregulated and activated in human RMS cell lines and patient samples. Inhibition of the Notch pathway by a γ-secretase inhibitor (GSI) decreased the in vitro proliferation of RMS cells. Knockdown of RBPJ expression by RNAi inhibited the anchorage-independent growth of RMS cells and the growth of xenografts in vivo. Additionally, overexpression of RBPJ promoted the anchorage-independent growth of RMS cells. Further, we revealed that RBPJ regulated the cell cycle in RMS xenograft tumors and decreased proliferation. Our findings suggest that RBPJ regulates the RMS growth, and that the inhibition of RBPJ may be an effective therapeutic approach for patients with RMS.
Conflict of interest statement
Figures
References
-
- Wachtel M, Schafer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat Rev. 2010;36:318–327. - PubMed
-
- Rodeberg D, Paidas C. Childhood rhabdomyosarcoma. Semin Pediatr Surg. 2006;15:57–62. - PubMed
-
- Wachtel M, Schafer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat Rev. 2010;36:318–327. - PubMed
-
- Perez EA, Kassira N, Cheung MC, Koniaris LG, Neville HL, et al. Rhabdomyosarcoma in Children: A SEER Population Based Study. J Surg Res. 2011;170:e243–e251. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

