Morphology, biophysical properties and protein-mediated fusion of archaeosomes
- PMID: 22792173
- PMCID: PMC3391208
- DOI: 10.1371/journal.pone.0039401
Morphology, biophysical properties and protein-mediated fusion of archaeosomes
Abstract
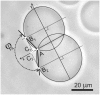
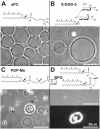
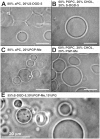
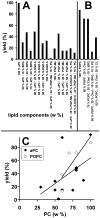
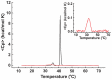
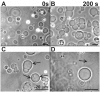
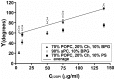
As variance from standard phospholipids of eubacteria and eukaryotes, archaebacterial diether phospholipids contain branched alcohol chains (phytanol) linked to glycerol exclusively with ether bonds. Giant vesicles (GVs) constituted of different species of archaebacterial diether phospholipids and glycolipids (archaeosomes) were prepared by electroformation and observed under a phase contrast and/or fluorescence microscope. Archaebacterial lipids and different mixtures of archaebacterial and standard lipids formed GVs which were analysed for size, yield and ability to adhere to each other due to the mediating effects of certain plasma proteins. GVs constituted of different proportions of archaeal or standard phosphatidylcholine were compared. In nonarchaebacterial GVs (in form of multilamellar lipid vesicles, MLVs) the main transition was detected at T(m) = 34. 2°C with an enthalpy of ΔH = 0.68 kcal/mol, whereas in archaebacterial GVs (MLVs) we did not observe the main phase transition in the range between 10 and 70°C. GVs constituted of archaebacterial lipids were subject to attractive interaction mediated by beta 2 glycoprotein I and by heparin. The adhesion constant of beta 2 glycoprotein I-mediated adhesion determined from adhesion angle between adhered GVs was in the range of 10(-8) J/m(2). In the course of protein mediated adhesion, lateral segregation of the membrane components and presence of thin tubular membranous structures were observed. The ability of archaebacterial diether lipids to combine with standard lipids in bilayers and their compatibility with adhesion-mediating molecules offer further evidence that archaebacterial lipids are appropriate for the design of drug carriers.
Conflict of interest statement
Figures



References
-
- Kates M, Wassef MK, Kushner DJ. Radioisotopic studies on the biosynthesis of the glyceryl diether lipids of Halobacterium cutirubrum. Can J Biochem. 1968;46:971–977. - PubMed
-
- Kates M, Deroo PW. Structure determination of the glycolipid sulphate from the extreme halophile Halobacterium cutirubrum. J Lipid Res. 1973;14:438–445. - PubMed
-
- Kates M. Kates M, Kushner DJ, Matheson AT, editors. Membrane lipids of Archaea. 1993. pp. 261–295. The Biochemistry of Archaea (Archaebacteria). Amsterdam, The Netherlands: Elsevier.
-
- Corcelli A, Lobasso S. Rainey FA, Oren A, editors. Characterization of lipids of halophilic Archaea. 2006. pp. 585–613. Methods in Microbiology–Extremophiles. Amsterdam, The Netherlands: Elsevier.
-
- Albers SV, van de Vossenberg JL, Driessen AJ, Konings WN. Adaptations of the archaeal cell membrane to heat stress. Front Biosci. 2000;5:813–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

