Blockage of spontaneous Ca2+ oscillation causes cell death in intraerythrocitic Plasmodium falciparum
- PMID: 22792177
- PMCID: PMC3391199
- DOI: 10.1371/journal.pone.0039499
Blockage of spontaneous Ca2+ oscillation causes cell death in intraerythrocitic Plasmodium falciparum
Abstract
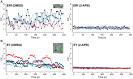
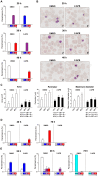
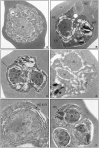
Malaria remains one of the world's most important infectious diseases and is responsible for enormous mortality and morbidity. Resistance to antimalarial drugs is a challenging problem in malaria control. Clinical malaria is associated with the proliferation and development of Plasmodium parasites in human erythrocytes. Especially, the development into the mature forms (trophozoite and schizont) of Plasmodium falciparum (P. falciparum) causes severe malaria symptoms due to a distinctive property, sequestration which is not shared by any other human malaria. Ca(2+) is well known to be a highly versatile intracellular messenger that regulates many different cellular processes. Cytosolic Ca(2+) increases evoked by extracellular stimuli are often observed in the form of oscillating Ca(2+) spikes (Ca(2+) oscillation) in eukaryotic cells. However, in lower eukaryotic and plant cells the physiological roles and the molecular mechanisms of Ca(2+) oscillation are poorly understood. Here, we showed the observation of the inositol 1,4,5-trisphospate (IP(3))-dependent spontaneous Ca(2+) oscillation in P. falciparum without any exogenous extracellular stimulation by using live cell fluorescence Ca(2+) imaging. Intraerythrocytic P. falciparum exhibited stage-specific Ca(2+) oscillations in ring form and trophozoite stages which were blocked by IP(3) receptor inhibitor, 2-aminoethyl diphenylborinate (2-APB). Analyses of parasitaemia and parasite size and electron micrograph of 2-APB-treated P. falciparum revealed that 2-APB severely obstructed the intraerythrocytic maturation, resulting in cell death of the parasites. Furthermore, we confirmed the similar lethal effect of 2-APB on the chloroquine-resistant strain of P. falciparum. To our best knowledge, we for the first time showed the existence of the spontaneous Ca(2+) oscillation in Plasmodium species and clearly demonstrated that IP(3)-dependent spontaneous Ca(2+) oscillation in P. falciparum is critical for the development of the blood stage of the parasites. Our results provide a novel concept that IP(3)/Ca(2+) signaling pathway in the intraerythrocytic malaria parasites is a promising target for antimalarial drug development.
Conflict of interest statement
Figures

References
-
- World Health Organization. World Malaria Report 2010. WHO. 2011.
-
- Haldar K, Mohandas N. Erythrocyte remodeling by malaria parasites. Curr Opin Hematol. 2007;14:203–209. doi: 10.1097/MOH.0b013e3280f31b2d. - DOI - PubMed
-
- Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. - DOI - PubMed
-
- Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–317. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

