HCV genotypes are differently prone to the development of resistance to linear and macrocyclic protease inhibitors
- PMID: 22792183
- PMCID: PMC3391197
- DOI: 10.1371/journal.pone.0039652
HCV genotypes are differently prone to the development of resistance to linear and macrocyclic protease inhibitors
Abstract
Background: Because of the extreme genetic variability of hepatitis C virus (HCV), we analyzed whether specific HCV-genotypes are differently prone to develop resistance to linear and macrocyclic protease-inhibitors (PIs).
Methods: The study includes 1568 NS3-protease sequences, isolated from PI-naive patients infected with HCV-genotypes 1a (N = 621), 1b (N = 474), 2 (N = 72), 3 (N = 268), 4 (N = 54) 5 (N = 6), and 6 (N = 73). Genetic-barrier was calculated as the sum of nucleotide-transitions (score = 1) and/or nucleotide-transversions (score = 2.5) required for drug-resistance-mutations emergence. Forty-three mutations associated with PIs-resistance were analyzed (36A/M/L/G-41R-43S/V-54A/S/V-55A-Q80K/R/L/H/G-109K-138T-155K/Q/T/I/M/S/G/L-156T/V/G/S-158I-168A/H/T/V/E/I/G/N/Y-170A/T-175L). Structural analyses on NS3-protease and on putative RNA-models have been also performed.
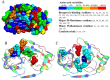
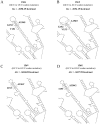
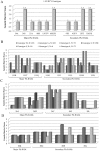
Results: Overall, NS3-protease was moderately conserved, with 85/181 (47.0%) amino-acids showing <1% variability. The catalytic-triad (H57-D81-S139) and 6/13 resistance-associated positions (Q41-F43-R109-R155-A156-V158) were fully conserved (variability <1%). Structural-analysis highlighted that most of the NS3-residues involved in drug-stabilization were highly conserved, while 7 PI-resistance residues, together with selected residues located in proximity of the PI-binding pocket, were highly variable among HCV-genotypes. Four resistance-mutations (80K/G-36L-175L) were found as natural polymorphisms in selected genotypes (80K present in 41.6% HCV-1a, 100% of HCV-5 and 20.6% HCV-6; 80G present in 94.4% HCV-2; 36L present in 100% HCV-3-5 and >94% HCV-2-4; 175L present in 100% HCV-1a-3-5 and >97% HCV-2-4). Furthermore, HCV-3 specifically showed non-conservative polymorphisms (R123T-D168Q) at two drug-interacting positions. Regardless of HCV-genotype, 13 PIs resistance-mutations were associated with low genetic-barrier, requiring only 1 nucleotide-substitution (41R-43S/V-54A-55A-80R-156V/T: score = 1; 54S-138T-156S/G-168E/H: score = 2.5). By contrast, by using HCV-1b as reference genotype, nucleotide-heterogeneity led to a lower genetic-barrier for the development of some drug-resistance-mutations in HCV-1a (36M-155G/I/K/M/S/T-170T), HCV-2 (36M-80K-155G/I/K/S/T-170T), HCV-3 (155G/I/K/M/S/T-170T), HCV-4-6 (155I/S/L), and HCV-5 (80G-155G/I/K/M/S/T).
Conclusions: The high degree of HCV genetic variability makes HCV-genotypes, and even subtypes, differently prone to the development of PIs resistance-mutations. Overall, this can account for different responsiveness of HCV-genotypes to PIs, with important clinical implications in tailoring individualized and appropriate regimens.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO) HCV Fact Sheet. Accessed 2012 Jun 15. 2011. World Health Organization (WHO) HCV Fact Sheet Available: http://www.who.int/mediacentre/factsheets/fs164/en/.
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358: 958–965. S0140673601061025 [pii] 2001. - PubMed
-
- Fried MW. Advances in therapy for chronic hepatitis C. Clin Liver Dis. 2001;5:1009–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

