Following ligand migration pathways from picoseconds to milliseconds in type II truncated hemoglobin from Thermobifida fusca
- PMID: 22792194
- PMCID: PMC3391200
- DOI: 10.1371/journal.pone.0039884
Following ligand migration pathways from picoseconds to milliseconds in type II truncated hemoglobin from Thermobifida fusca
Abstract
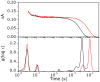
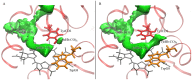
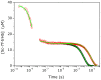
CO recombination kinetics has been investigated in the type II truncated hemoglobin from Thermobifida fusca (Tf-trHb) over more than 10 time decades (from 1 ps to ∼100 ms) by combining femtosecond transient absorption, nanosecond laser flash photolysis and optoacoustic spectroscopy. Photolysis is followed by a rapid geminate recombination with a time constant of ∼2 ns representing almost 60% of the overall reaction. An additional, small amplitude geminate recombination was identified at ∼100 ns. Finally, CO pressure dependent measurements brought out the presence of two transient species in the second order rebinding phase, with time constants ranging from ∼3 to ∼100 ms. The available experimental evidence suggests that the two transients are due to the presence of two conformations which do not interconvert within the time frame of the experiment. Computational studies revealed that the plasticity of protein structure is able to define a branched pathway connecting the ligand binding site and the solvent. This allowed to build a kinetic model capable of describing the complete time course of the CO rebinding kinetics to Tf-trHb.
Conflict of interest statement
Figures






References
-
- Martin JL, Vos MH. Femtosecond biology. Annu Rev Bioph Biomol Struct. 1992;21:199–222. - PubMed
-
- Vos MH, Martin JL. Femtosecond processes in protein. Biochim Biophys Acta. 1999;1411:1–20. - PubMed
-
- Vos MH. Ultrafast dynamics of ligands within heme proteins. Biochim Biophys Acta. 2008;1777:15–31. - PubMed
-
- Ansari A, Jones CM, Henry ER, Hofrichter J, Eaton WA. Conformational relaxation and ligand binding in myoglobin. Biochemistry. 1994;33:5128–5145–2226. - PubMed
-
- Olson JS, Phillips GN., Jr Kinetics pathway and barriers for ligand binding myoglobin. J. Biol. Chem. 2008;271:17593–17596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

