The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury
- PMID: 22792197
- PMCID: PMC3391230
- DOI: 10.1371/journal.pone.0039934
The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury
Abstract
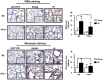
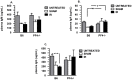
The robust inflammatory response that occurs during ischemia reperfusion (IR) injury recruits factors from both the innate and adaptive immune systems. However the contribution of platelets and their products such as Platelet Factor 4 (PF4; CXCL4), during the pathogenesis of IR injury has not been thoroughly investigated. We show that a deficiency in PF4 protects mice from local and remote tissue damage after 30 minutes of mesenteric ischemia and 3 hours of reperfusion in PF4-/- mice compared to control B6 mice. This protection was independent from Ig or complement deposition in the tissues. However, neutrophil and monocyte infiltration were decreased in the lungs of PF4-/- mice compared with B6 control mice. Platelet-depleted B6 mice transfused with platelets from PF4-/- mice displayed reduced tissue damage compared with controls. In contrast, transfusion of B6 platelets into platelet depleted PF4-/- mice reconstituted damage in both intestine and lung tissues. We also show that PF4 may modulate the release of IgA. Interestingly, we show that PF4 expression on intestinal epithelial cells is increased after IR at both the mRNA and protein levels. In conclusion, these findings demonstrate that may PF4 represent an important mediator of local and remote tissue damage.
Conflict of interest statement
Figures





Similar articles
-
Platelet-associated CD40/CD154 mediates remote tissue damage after mesenteric ischemia/reperfusion injury.PLoS One. 2012;7(2):e32260. doi: 10.1371/journal.pone.0032260. Epub 2012 Feb 27. PLoS One. 2012. PMID: 22384195 Free PMC article.
-
Inhibition of Syk activity by R788 in platelets prevents remote lung tissue damage after mesenteric ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2012 Jun 15;302(12):G1416-22. doi: 10.1152/ajpgi.00026.2012. Epub 2012 Apr 5. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22492694
-
Platelets orchestrate remote tissue damage after mesenteric ischemia-reperfusion.Am J Physiol Gastrointest Liver Physiol. 2012 Apr 15;302(8):G888-97. doi: 10.1152/ajpgi.00499.2011. Epub 2012 Feb 2. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22301111
-
Complement depletion protects lupus-prone mice from ischemia-reperfusion-initiated organ injury.Am J Physiol Gastrointest Liver Physiol. 2013 Feb 1;304(3):G283-92. doi: 10.1152/ajpgi.00371.2012. Epub 2012 Oct 25. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23104558
-
Membrane lipid interactions in intestinal ischemia/reperfusion-induced Injury.Clin Immunol. 2014 Jul;153(1):228-40. doi: 10.1016/j.clim.2014.04.018. Epub 2014 May 9. Clin Immunol. 2014. PMID: 24814240 Free PMC article. Review.
Cited by
-
Evaluation of Pulmonary Reperfusion Injury in Rats Undergoing Mesenteric Ischemia and Reperfusion and Protective Effect of Postconditioning on this Process.Braz J Cardiovasc Surg. 2015 Sep-Oct;30(5):533-7. doi: 10.5935/1678-9741.20150067. Braz J Cardiovasc Surg. 2015. PMID: 26735599 Free PMC article.
-
CXCL4 is a novel inducer of human Th17 cells and correlates with IL-17 and IL-22 in psoriatic arthritis.Eur J Immunol. 2018 Mar;48(3):522-531. doi: 10.1002/eji.201747195. Epub 2018 Jan 15. Eur J Immunol. 2018. PMID: 29193036 Free PMC article.
-
Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice.Blood Adv. 2018 Mar 27;2(6):638-648. doi: 10.1182/bloodadvances.2017011817. Blood Adv. 2018. PMID: 29563120 Free PMC article.
-
NGS nominated CELA1, HSPG2, and KCNK5 as candidate genes for predisposition to Balkan endemic nephropathy.Biomed Res Int. 2014;2014:920723. doi: 10.1155/2014/920723. Epub 2014 May 15. Biomed Res Int. 2014. PMID: 24949484 Free PMC article.
-
Megakaryocytes as immune cells.J Leukoc Biol. 2019 Jun;105(6):1111-1121. doi: 10.1002/JLB.MR0718-261RR. Epub 2019 Jan 15. J Leukoc Biol. 2019. PMID: 30645026 Free PMC article. Review.
References
-
- Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249:889–899. - PubMed
-
- Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. - PubMed
-
- Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

