Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors
- PMID: 22792255
- PMCID: PMC3390346
- DOI: 10.1371/journal.pone.0040255
Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors
Abstract
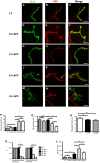
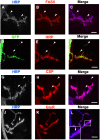
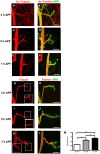
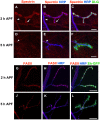
Larval motor neurons remodel during Drosophila neuro-muscular junction dismantling at metamorphosis. In this study, we describe the motor neuron retraction as opposed to degeneration based on the early disappearance of β-Spectrin and the continuing presence of Tubulin. By blocking cell dynamics with a dominant-negative form of Dynamin, we show that phagocytes have a key role in this process. Importantly, we show the presence of peripheral glial cells close to the neuro-muscular junction that retracts before the motor neuron. We show also that in muscle, expression of EcR-B1 encoding the steroid hormone receptor required for postsynaptic dismantling, is under the control of the ftz-f1/Hr39 orphan nuclear receptor pathway but not the TGF-β signaling pathway. In the motor neuron, activation of EcR-B1 expression by the two parallel pathways (TGF-β signaling and nuclear receptor) triggers axon retraction. We propose that a signal from a TGF-β family ligand is produced by the dismantling muscle (postsynapse compartment) and received by the motor neuron (presynaptic compartment) resulting in motor neuron retraction. The requirement of the two pathways in the motor neuron provides a molecular explanation for the instructive role of the postsynapse degradation on motor neuron retraction. This mechanism insures the temporality of the two processes and prevents motor neuron pruning before postsynaptic degradation.
Conflict of interest statement
Figures
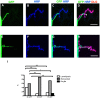
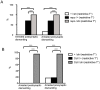
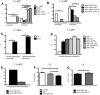
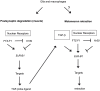
Similar articles
-
Two-factor specification of apoptosis: TGF-β signaling acts cooperatively with ecdysone signaling to induce cell- and stage-specific apoptosis of larval neurons during metamorphosis in Drosophila melanogaster.Apoptosis. 2019 Dec;24(11-12):972-989. doi: 10.1007/s10495-019-01574-4. Apoptosis. 2019. PMID: 31641960
-
ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling.Nat Neurosci. 2011 Jan;14(1):37-44. doi: 10.1038/nn.2700. Epub 2010 Dec 5. Nat Neurosci. 2011. PMID: 21131955
-
Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand.Curr Biol. 2012 Oct 9;22(19):1831-8. doi: 10.1016/j.cub.2012.07.063. Epub 2012 Sep 6. Curr Biol. 2012. PMID: 22959350 Free PMC article.
-
Transforming growth factor-β signaling in motor neuron diseases.Curr Mol Med. 2011 Feb;11(1):48-56. doi: 10.2174/156652411794474356. Curr Mol Med. 2011. PMID: 21189118 Review.
-
Nuclear receptors and Drosophila neuronal remodeling.Biochim Biophys Acta. 2015 Feb;1849(2):187-95. doi: 10.1016/j.bbagrm.2014.05.024. Epub 2014 Jun 2. Biochim Biophys Acta. 2015. PMID: 24882358 Review.
Cited by
-
Chemokine-like Orion is involved in the transformation of glial cells into phagocytes in different developmental neuronal remodeling paradigms.Development. 2023 Oct 1;150(19):dev201633. doi: 10.1242/dev.201633. Epub 2023 Oct 2. Development. 2023. PMID: 37767633 Free PMC article.
-
Subtype-specific neuronal remodeling during Drosophila metamorphosis.Fly (Austin). 2013 Apr-Jun;7(2):78-86. doi: 10.4161/fly.23969. Epub 2013 Apr 1. Fly (Austin). 2013. PMID: 23579264 Free PMC article.
-
Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila.Dev Biol. 2014 Jan 15;385(2):253-62. doi: 10.1016/j.ydbio.2013.11.003. Epub 2013 Nov 15. Dev Biol. 2014. PMID: 24247008 Free PMC article.
-
Establishment of a novel axon pruning model of Drosophila motor neuron.Biol Open. 2023 Jan 1;12(1):bio059535. doi: 10.1242/bio.059535. Epub 2023 Jan 6. Biol Open. 2023. PMID: 36606515 Free PMC article.
-
Shep regulates Drosophila neuronal remodeling by controlling transcription of its chromatin targets.Development. 2018 Jan 3;145(1):dev154047. doi: 10.1242/dev.154047. Development. 2018. PMID: 29158441 Free PMC article.
References
-
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annual review of neuroscience. 2005;28:127–156. - PubMed
-
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. - PubMed
-
- Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. - PubMed
-
- Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–3642. - PubMed
-
- Zheng X, Wang J, Haerry TE, Wu AY, Martin J, et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

