Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation
- PMID: 22792275
- PMCID: PMC3392250
- DOI: 10.1371/journal.pone.0040314
Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation
Abstract
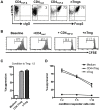
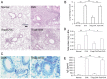
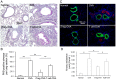
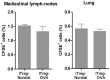
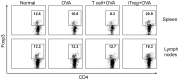
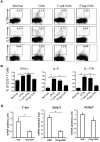
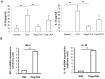
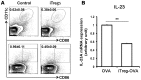
Both nature and induced regulatory T (Treg) lymphocytes are potent regulators of autoimmune and allergic disorders. Defects in endogenous Treg cells have been reported in patients with allergic asthma, suggesting that disrupted Treg cell-mediated immunological regulation may play an important role in airway allergic inflammation. In order to determine whether adoptive transfer of induced Treg cells generated in vitro can be used as an effective therapeutic approach to suppress airway allergic inflammation, exogenously induced Treg cells were infused into ovalbumin-sensitized mice prior to or during intranasal ovalbumin challenge. The results showed that adoptive transfer of induced Treg cells prior to allergen challenge markedly reduced airway hyperresponsiveness, eosinophil recruitment, mucus hyper-production, airway remodeling, and IgE levels. This effect was associated with increase of Treg cells (CD4(+)FoxP3(+)) and decrease of dendritic cells in the draining lymph nodes, and with reduction of Th1, Th2, and Th17 cell response as compared to the controls. Moreover, adoptive transfer of induced Treg cells during allergen challenge also effectively attenuate airway inflammation and improve airway function, which are comparable to those by natural Treg cell infusion. Therefore, adoptive transfer of in vitro induced Treg cells may be a promising therapeutic approach to prevent and treat severe asthma.
Conflict of interest statement
Figures
Similar articles
-
Adalimumab ameliorates OVA-induced airway inflammation in mice: Role of CD4(+) CD25(+) FOXP3(+) regulatory T-cells.Eur J Pharmacol. 2016 Sep 5;786:100-108. doi: 10.1016/j.ejphar.2016.06.002. Epub 2016 Jun 2. Eur J Pharmacol. 2016. PMID: 27262379
-
Parasitic nematode-induced CD4+Foxp3+T cells can ameliorate allergic airway inflammation.PLoS Negl Trop Dis. 2014 Dec 18;8(12):e3410. doi: 10.1371/journal.pntd.0003410. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25522145 Free PMC article.
-
Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells.J Allergy Clin Immunol. 2012 Jun;129(6):1656-65.e3. doi: 10.1016/j.jaci.2012.02.050. Epub 2012 May 5. J Allergy Clin Immunol. 2012. PMID: 22564681
-
Regulatory T cell migration during an immune response.Trends Immunol. 2012 Apr;33(4):174-80. doi: 10.1016/j.it.2012.01.002. Epub 2012 Feb 2. Trends Immunol. 2012. PMID: 22305714 Free PMC article. Review.
-
LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation.Int Immunopharmacol. 2011 Jul;11(7):827-32. doi: 10.1016/j.intimp.2011.01.034. Epub 2011 Feb 12. Int Immunopharmacol. 2011. PMID: 21320637 Free PMC article. Review.
Cited by
-
Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism.J Clin Invest. 2013 Mar;123(3):1216-28. doi: 10.1172/JCI65351. Epub 2013 Feb 8. J Clin Invest. 2013. PMID: 23391720 Free PMC article.
-
Excretory/Secretory Products from Schistosoma japonicum Eggs Alleviate Ovalbumin-Induced Allergic Airway Inflammation.PLoS Negl Trop Dis. 2023 Oct 3;17(10):e0011625. doi: 10.1371/journal.pntd.0011625. eCollection 2023 Oct. PLoS Negl Trop Dis. 2023. PMID: 37788409 Free PMC article.
-
Tregs in Autoimmune Uveitis.Adv Exp Med Biol. 2021;1278:205-227. doi: 10.1007/978-981-15-6407-9_11. Adv Exp Med Biol. 2021. PMID: 33523450
-
Tolerogenic nanoparticles induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis.Sci Adv. 2022 Nov 25;8(47):eabo5284. doi: 10.1126/sciadv.abo5284. Epub 2022 Nov 25. Sci Adv. 2022. PMID: 36427299 Free PMC article.
-
Dichotomous role of TGF-β controls inducible regulatory T-cell fate in allergic airway disease through Smad3 and TGF-β-activated kinase 1.J Allergy Clin Immunol. 2020 Mar;145(3):933-946.e4. doi: 10.1016/j.jaci.2019.09.032. Epub 2019 Oct 15. J Allergy Clin Immunol. 2020. PMID: 31626843 Free PMC article.
References
-
- Moorman JE, Rudd RA, Johnson CA, King M, Minor P, et al. National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56:1–54. - PubMed
-
- Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. - PubMed
-
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19:676–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

