Binding of hnRNP H and U2AF65 to respective G-codes and a poly-uridine tract collaborate in the N50-5'ss selection of the REST N exon in H69 cells
- PMID: 22792276
- PMCID: PMC3390395
- DOI: 10.1371/journal.pone.0040315
Binding of hnRNP H and U2AF65 to respective G-codes and a poly-uridine tract collaborate in the N50-5'ss selection of the REST N exon in H69 cells
Abstract
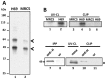
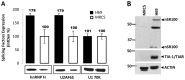
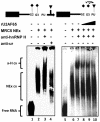
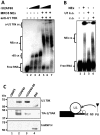
The splicing of the N exon in the pre-mRNA coding for the RE1-silencing transcription factor (REST) results in a truncated protein that modifies the expression pattern of some of its target genes. A weak 3'ss, three alternative 5'ss (N4-, N50-, and N62-5'ss) and a variety of putative target sites for splicing regulatory proteins are found around the N exon; two GGGG codes (G2-G3) and a poly-Uridine tract (N-PU) are found in front of the N50-5'ss. In this work we analyzed some of the regulatory factors and elements involved in the preferred selection of the N50-5'ss (N50 activation) in the small cell lung cancer cell line H69. Wild type and mutant N exon/β-globin minigenes recapitulated N50 exon splicing in H69 cells, and showed that the N-PU and the G2-G3 elements are required for N50 exon splicing. Biochemical and knockdown experiments identified these elements as U2AF65 and hnRNP H targets, respectively, and that they are also required for N50 exon activation. Compared to normal MRC5 cells, and in keeping with N50 exon activation, U2AF65, hnRNP H and other splicing factors were highly expressed in H69 cells. CLIP experiments revealed that hnRNP H RNA-binding occurs first and is a prerequisite for U2AF65 RNA binding, and EMSA and CLIP experiments suggest that U2AF65-RNA recognition displaces hnRNP H and helps to recruit other splicing factors (at least U1 70K) to the N50-5'ss. Our results evidenced novel hnRNP H and U2AF65 functions: respectively, U2AF65-recruiting to a 5'ss in humans and the hnRNP H-displacing function from two juxtaposed GGGG codes.
Conflict of interest statement
Figures




References
-
- Venables JP. Aberrant and alternative splicing in cancer. Cancer Res. 2004;64:7647–7654. - PubMed
-
- Kirschbaum-Slager N, Lopes GM, Galante PA, Riggins GJ, de Souza SJ. Splicing factors are differentially expressed in tumors. Genet Mol Res. 2004;3:512–520. - PubMed
-
- Coulson JM, Fiskerstrand CE, Woll PJ, Quinn JP. Arginine vasopressin promoter regulation is mediated by a neuron-restrictive silencer element in small cell lung cancer. Cancer Res. 1999;59:5123–5127. - PubMed
-
- Coulson JM, Edgson JL, Woll PJ, Quinn JP. A splice variant of the neuron-restrictive silencer factor repressor is expressed in small cell lung cancer: a potential role in derepression of neuroendocrine genes and a useful clinical marker. Cancer Res. 2000;60:1840–1844. - PubMed
-
- Palm K, Metsis M, Timmusk T. Neuron-specific splicing of zinc finger transcription factor REST/NRSF/XBR is frequent in neuroblastomas and conserved in human, mouse and rat. Brain Res Mol Brain Res. 1999;72:30–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

