Characterisation of the first enzymes committed to lysine biosynthesis in Arabidopsis thaliana
- PMID: 22792278
- PMCID: PMC3390394
- DOI: 10.1371/journal.pone.0040318
Characterisation of the first enzymes committed to lysine biosynthesis in Arabidopsis thaliana
Abstract
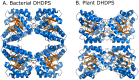
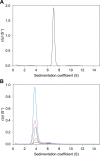
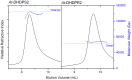
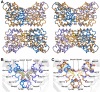
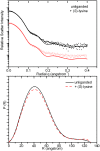
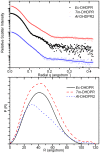
In plants, the lysine biosynthetic pathway is an attractive target for both the development of herbicides and increasing the nutritional value of crops given that lysine is a limiting amino acid in cereals. Dihydrodipicolinate synthase (DHDPS) and dihydrodipicolinate reductase (DHDPR) catalyse the first two committed steps of lysine biosynthesis. Here, we carry out for the first time a comprehensive characterisation of the structure and activity of both DHDPS and DHDPR from Arabidopsis thaliana. The A. thaliana DHDPS enzyme (At-DHDPS2) has similar activity to the bacterial form of the enzyme, but is more strongly allosterically inhibited by (S)-lysine. Structural studies of At-DHDPS2 show (S)-lysine bound at a cleft between two monomers, highlighting the allosteric site; however, unlike previous studies, binding is not accompanied by conformational changes, suggesting that binding may cause changes in protein dynamics rather than large conformation changes. DHDPR from A. thaliana (At-DHDPR2) has similar specificity for both NADH and NADPH during catalysis, and has tighter binding of substrate than has previously been reported. While all known bacterial DHDPR enzymes have a tetrameric structure, analytical ultracentrifugation, and scattering data unequivocally show that At-DHDPR2 exists as a dimer in solution. The exact arrangement of the dimeric protein is as yet unknown, but ab initio modelling of x-ray scattering data is consistent with an elongated structure in solution, which does not correspond to any of the possible dimeric pairings observed in the X-ray crystal structure of DHDPR from other organisms. This increased knowledge of the structure and function of plant lysine biosynthetic enzymes will aid future work aimed at improving primary production.
Conflict of interest statement
Figures

Similar articles
-
Structure and Function of Cyanobacterial DHDPS and DHDPR.Sci Rep. 2016 Nov 15;6:37111. doi: 10.1038/srep37111. Sci Rep. 2016. PMID: 27845445 Free PMC article.
-
Plant DHDPR forms a dimer with unique secondary structure features that preclude higher-order assembly.Biochem J. 2018 Jan 5;475(1):137-150. doi: 10.1042/BCJ20170709. Biochem J. 2018. PMID: 29187521
-
The structure of dihydrodipicolinate reductase (DapB) from Mycobacterium tuberculosis in three crystal forms.Acta Crystallogr D Biol Crystallogr. 2010 Jan;66(Pt 1):61-72. doi: 10.1107/S0907444909043960. Epub 2009 Dec 21. Acta Crystallogr D Biol Crystallogr. 2010. PMID: 20057050
-
Dihydrodipicolinate Synthase: Structure, Dynamics, Function, and Evolution.Subcell Biochem. 2017;83:271-289. doi: 10.1007/978-3-319-46503-6_10. Subcell Biochem. 2017. PMID: 28271480 Review.
-
Molecular evolution of an oligomeric biocatalyst functioning in lysine biosynthesis.Biophys Rev. 2018 Apr;10(2):153-162. doi: 10.1007/s12551-017-0350-y. Epub 2017 Dec 5. Biophys Rev. 2018. PMID: 29204887 Free PMC article. Review.
Cited by
-
Medicago truncatula dihydrodipicolinate synthase (DHDPS) enzymes display novel regulatory properties.Plant Mol Biol. 2013 Mar;81(4-5):401-15. doi: 10.1007/s11103-013-0008-5. Epub 2013 Jan 18. Plant Mol Biol. 2013. PMID: 23329373
-
Metabolite Profile of Xylem Sap in Cotton Seedlings Is Changed by K Deficiency.Front Plant Sci. 2020 Dec 10;11:592591. doi: 10.3389/fpls.2020.592591. eCollection 2020. Front Plant Sci. 2020. PMID: 33362821 Free PMC article.
-
Genome-Wide Analysis of the Lysine Biosynthesis Pathway Network during Maize Seed Development.PLoS One. 2016 Feb 1;11(2):e0148287. doi: 10.1371/journal.pone.0148287. eCollection 2016. PLoS One. 2016. PMID: 26829553 Free PMC article.
-
Structural, kinetic and computational investigation of Vitis vinifera DHDPS reveals new insight into the mechanism of lysine-mediated allosteric inhibition.Plant Mol Biol. 2013 Mar;81(4-5):431-46. doi: 10.1007/s11103-013-0014-7. Epub 2013 Jan 26. Plant Mol Biol. 2013. PMID: 23354837
-
Towards novel herbicide modes of action by inhibiting lysine biosynthesis in plants.Elife. 2021 Jul 27;10:e69444. doi: 10.7554/eLife.69444. Elife. 2021. PMID: 34313586 Free PMC article.
References
-
- Galili G. New insights into the regulation and functional significance of lysine metabolism in plants, Annual Review of Plant Biology 53, 27–43. 2002. - PubMed
-
- Jander G, Joshi V. Recent Progress in Deciphering the Biosynthesis of Aspartate-Derived Amino Acids in Plants, Molecular Plant 3, 54–65. 2010. - PubMed
-
- Frizzi A, Huang S, Gilbertson LA, Armstrong TA, Luethy MH, et al. Modifying lysine biosynthesis and catabolism in corn with a single bifunctional expression/silencing transgene cassette, Plant Biotechnol J 6, 13–21. 2008. - PubMed
-
- Hudson A O, Bless C, Macedo P, Chatterjee S P, Singh B K, et al. Biosynthesis of lysine in plants: evidence for a variant of the known bacterial pathways, Biochim Biophys Acta 1721, 27–36. 2005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

