Dysregulation of suppressor of cytokine signaling 3 in keratinocytes causes skin inflammation mediated by interleukin-20 receptor-related cytokines
- PMID: 22792286
- PMCID: PMC3390387
- DOI: 10.1371/journal.pone.0040343
Dysregulation of suppressor of cytokine signaling 3 in keratinocytes causes skin inflammation mediated by interleukin-20 receptor-related cytokines
Abstract
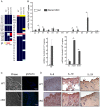
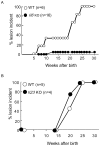
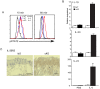
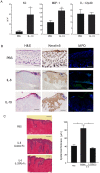
Homeostatic regulation of epidermal keratinocytes is controlled by the local cytokine milieu. However, a role for suppressor of cytokine signaling (SOCS), a negative feedback regulator of cytokine networks, in skin homeostasis remains unclear. Keratinocyte specific deletion of Socs3 (Socs3 cKO) caused severe skin inflammation with hyper-production of IgE, epidermal hyperplasia, and S100A8/9 expression, although Socs1 deletion caused no inflammation. The inflamed skin showed constitutive STAT3 activation and up-regulation of IL-6 and IL-20 receptor (IL-20R) related cytokines, IL-19, IL-20 and IL-24. Disease development was rescued by deletion of the Il6 gene, but not by the deletion of Il23, Il4r, or Rag1 genes. The expression of IL-6 in Socs3 cKO keratinocytes increased expression of IL-20R-related cytokines that further facilitated STAT3 hyperactivation, epidermal hyperplasia and neutrophilia. These results demonstrate that skin homeostasis is strictly regulated by the IL-6-STAT3-SOCS3 axis. Moreover, the SOCS3-mediated negative feedback loop in keratinocytes has a critical mechanistic role in the prevention of skin inflammation caused by hyperactivation of STAT3.
Conflict of interest statement
Figures



Similar articles
-
Human primary keratinocytes show restricted ability to up-regulate suppressor of cytokine signaling (SOCS)3 protein compared with autologous macrophages.J Biol Chem. 2012 Mar 23;287(13):9923-9930. doi: 10.1074/jbc.M111.280370. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294695 Free PMC article.
-
Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1.J Immunol. 2002 Jul 1;169(1):434-42. doi: 10.4049/jimmunol.169.1.434. J Immunol. 2002. PMID: 12077274
-
TGF-beta promotes Th17 cell development through inhibition of SOCS3.J Immunol. 2009 Jul 1;183(1):97-105. doi: 10.4049/jimmunol.0801986. Epub 2009 Jun 17. J Immunol. 2009. PMID: 19535626 Free PMC article.
-
SOCS1 and SOCS3 as key checkpoint molecules in the immune responses associated to skin inflammation and malignant transformation.Front Immunol. 2024 Jun 21;15:1393799. doi: 10.3389/fimmu.2024.1393799. eCollection 2024. Front Immunol. 2024. PMID: 38975347 Free PMC article. Review.
-
The role of SOCS3 in modulating leukaemia inhibitory factor signalling during murine placental development.J Reprod Immunol. 2008 Jan;77(1):1-6. doi: 10.1016/j.jri.2007.02.003. Epub 2007 Apr 3. J Reprod Immunol. 2008. PMID: 17408753 Free PMC article. Review.
Cited by
-
SOCS3 revisited: a broad regulator of disease, now ready for therapeutic use?Cell Mol Life Sci. 2016 Sep;73(17):3323-36. doi: 10.1007/s00018-016-2234-x. Epub 2016 May 2. Cell Mol Life Sci. 2016. PMID: 27137184 Free PMC article. Review.
-
Lack of association between SOCS3 and SOCS7 polymorphisms and psoriasis.Immun Inflamm Dis. 2022 Oct;10(10):e695. doi: 10.1002/iid3.695. Immun Inflamm Dis. 2022. PMID: 36169255 Free PMC article.
-
RNase 7 and Th cytokines synergistically increase the secretion of interleukin-6 from keratinocytes.Sci Rep. 2025 Jun 3;15(1):19396. doi: 10.1038/s41598-025-04403-8. Sci Rep. 2025. PMID: 40461723 Free PMC article.
-
SOCS3 Expression by Thymic Stromal Cells Is Required for Normal T Cell Development.Front Immunol. 2021 Mar 18;12:642173. doi: 10.3389/fimmu.2021.642173. eCollection 2021. Front Immunol. 2021. PMID: 33815395 Free PMC article.
-
A Novel STAT3 Mutation in a Patient with Hyper-IgE Syndrome Diagnosed with a Severe Necrotizing Pulmonary Infection.J Asthma Allergy. 2021 Mar 12;14:219-227. doi: 10.2147/JAA.S295516. eCollection 2021. J Asthma Allergy. 2021. PMID: 33737815 Free PMC article.
References
-
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. - PubMed
-
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. - PubMed
-
- Zhu BM, Ishida Y, Robinson GW, Pacher-Zavisin M, Yoshimura A, et al. SOCS3 negatively regulates the gp130-STAT3 pathway in mouse skin wound healing. J Invest Dermatol. 2008;128:1821–1829. - PubMed
-
- Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–4152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

