Intrathecal, polyspecific antiviral immune response in oligoclonal band negative multiple sclerosis
- PMID: 22792316
- PMCID: PMC3392215
- DOI: 10.1371/journal.pone.0040431
Intrathecal, polyspecific antiviral immune response in oligoclonal band negative multiple sclerosis
Abstract
Background: Oligoclonal bands (OCB) are detected in the cerebrospinal fluid (CSF) in more than 95% of patients with multiple sclerosis (MS) in the Western hemisphere. Here we evaluated the intrathecal, polyspecific antiviral immune response as a potential diagnostic CSF marker for OCB-negative MS patients.
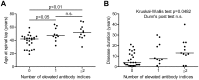
Methodology/principal findings: We tested 46 OCB-negative German patients with paraclinically well defined, definite MS. Sixteen OCB-negative patients with a clear diagnosis of other autoimmune CNS disorders and 37 neurological patients without evidence for autoimmune CNS inflammation served as control groups. Antibodies against measles, rubella, varicella zoster and herpes simplex virus in paired serum and CSF samples were determined by ELISA, and virus-specific immunoglobulin G antibody indices were calculated. An intrathecal antibody synthesis against at least one neurotropic virus was detected in 8 of 26 (31%) patients with relapsing-remitting MS, 8 of 12 (67%) with secondary progressive MS and 5 of 8 (63%) with primary progressive MS, in 3 of 16 (19%) CNS autoimmune and 3 of 37 (8%) non-autoimmune control patients. Antibody synthesis against two or more viruses was found in 11 of 46 (24%) MS patients but in neither of the two control groups. On average, MS patients with a positive antiviral immune response were older and had a longer disease duration than those without.
Conclusion: Determination of the intrathecal, polyspecific antiviral immune response may allow to establish a CSF-supported diagnosis of MS in OCB-negative patients when two or more of the four virus antibody indices are elevated.
Conflict of interest statement
Figures

Similar articles
-
Oligoclonal restriction of antiviral immunoreaction in oligoclonal band-negative MS patients.Acta Neurol Scand. 2015 Jun;131(6):381-8. doi: 10.1111/ane.12350. Epub 2014 Nov 17. Acta Neurol Scand. 2015. PMID: 25402869
-
MRZH reaction increases sensitivity for intrathecal IgG synthesis in IgG Oligoclonal band negative Multiple Sclerosis patients.J Neuroimmunol. 2016 Nov 15;300:30-35. doi: 10.1016/j.jneuroim.2016.10.001. Epub 2016 Oct 5. J Neuroimmunol. 2016. PMID: 27806873
-
The MRZ reaction in primary progressive multiple sclerosis.Fluids Barriers CNS. 2017 Feb 7;14(1):2. doi: 10.1186/s12987-016-0049-7. Fluids Barriers CNS. 2017. PMID: 28166789 Free PMC article.
-
The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature.J Neurol. 2017 Mar;264(3):453-466. doi: 10.1007/s00415-016-8360-4. Epub 2016 Dec 22. J Neurol. 2017. PMID: 28005176 Review.
-
The elusive nature of the oligoclonal bands in multiple sclerosis.J Neurol. 2024 Jan;271(1):116-124. doi: 10.1007/s00415-023-12081-7. Epub 2023 Nov 9. J Neurol. 2024. PMID: 37945762 Review.
Cited by
-
Atypical adult-onset subacute sclerosing panencephalitis.Acta Clin Croat. 2020 Sep;59(3):543-548. doi: 10.20471/acc.2020.59.03.21. Acta Clin Croat. 2020. PMID: 34177067 Free PMC article.
-
Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients.J Neuroinflammation. 2022 Jan 20;19(1):19. doi: 10.1186/s12974-021-02339-0. J Neuroinflammation. 2022. PMID: 35057809 Free PMC article.
-
The utility of cerebrospinal fluid analysis in patients with multiple sclerosis.Nat Rev Neurol. 2013 May;9(5):267-76. doi: 10.1038/nrneurol.2013.41. Epub 2013 Mar 26. Nat Rev Neurol. 2013. PMID: 23528543 Review.
-
[Cerebrospinal fluid diagnostics in multiple sclerosis].Nervenarzt. 2016 Dec;87(12):1282-1287. doi: 10.1007/s00115-016-0220-z. Nervenarzt. 2016. PMID: 27649986 Review. German.
-
[Clinically isolated syndrome].Nervenarzt. 2013 Oct;84(10):1247-59. doi: 10.1007/s00115-013-3845-1. Nervenarzt. 2013. PMID: 24081277 Review. German.
References
-
- Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler. 1998;4:111–117. - PubMed
-
- Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37:1153–1160. - PubMed
-
- Bednarova J, Stourac P, Adam P. Relevance of immunological variables in neuroborreliosis and multiple sclerosis. Acta Neurol Scand. 2005;112:97–102. - PubMed
-
- Jarius S, Eichhorn P, Jacobi C, Wildemann B, Wick M, et al. The intrathecal, polyspecific antiviral immune response: Specific for MS or a general marker of CNS autoimmunity? J Neurol Sci. 2009;280:98–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

