Participant informed consent in cluster randomized trials: review
- PMID: 22792319
- PMCID: PMC3391275
- DOI: 10.1371/journal.pone.0040436
Participant informed consent in cluster randomized trials: review
Abstract
Background: The Nuremberg code defines the general ethical framework of medical research with participant consent as its cornerstone. In cluster randomized trials (CRT), obtaining participant informed consent raises logistic and methodologic concerns. First, with randomization of large clusters such as geographical areas, obtaining individual informed consent may be impossible. Second, participants in randomized clusters cannot avoid certain interventions, which implies that participant informed consent refers only to data collection, not administration of an intervention. Third, complete participant information may be a source of selection bias, which then raises methodological concerns. We assessed whether participant informed consent was required in such trials, which type of consent was required, and whether the trial was at risk of selection bias because of the very nature of participant information.
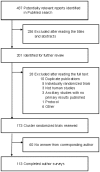
Methods and findings: We systematically reviewed all reports of CRT published in MEDLINE in 2008 and surveyed corresponding authors regarding the nature of the informed consent and the process of participant inclusion. We identified 173 reports and obtained an answer from 113 authors (65.3%). In total, 23.7% of the reports lacked information on ethics committee approval or participant consent, 53.1% of authors declared that participant consent was for data collection only and 58.5% that the group allocation was not specified for participants. The process of recruitment (chronology of participant recruitment with regard to cluster randomization) was rarely reported, and we estimated that only 56.6% of the trials were free of potential selection bias.
Conclusions: For CRTs, the reporting of ethics committee approval and participant informed consent is less than optimal. Reports should describe whether participants consented for administration of an intervention and/or data collection. Finally, the process of participant recruitment should be fully described (namely, whether participants were informed of the allocation group before being recruited) for a better appraisal of the risk of selection bias.
Conflict of interest statement
Figures
References
-
- Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. London: Arnold. 2000.
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess. 1999;3:iii–92. - PubMed
-
- Hayes RJ, Alexander ND, Bennett S, Cousens SN. Design and analysis issues in cluster-randomized trials of interventions against infectious diseases. Stat Methods Med Res. 2000;9:95–116. - PubMed
-
- Hayes RJ, Moulton LH. Cluster randomised trials. New York: chapman & Hall/CRC. 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials