NR4A1 (Nur77) mediates thyrotropin-releasing hormone-induced stimulation of transcription of the thyrotropin β gene: analysis of TRH knockout mice
- PMID: 22792320
- PMCID: PMC3392219
- DOI: 10.1371/journal.pone.0040437
NR4A1 (Nur77) mediates thyrotropin-releasing hormone-induced stimulation of transcription of the thyrotropin β gene: analysis of TRH knockout mice
Abstract
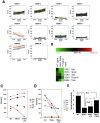
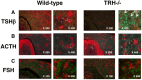
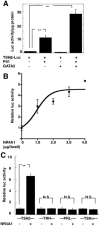
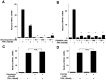
Thyrotropin-releasing hormone (TRH) is a major stimulator of thyrotropin-stimulating hormone (TSH) synthesis in the anterior pituitary, though precisely how TRH stimulates the TSHβ gene remains unclear. Analysis of TRH-deficient mice differing in thyroid hormone status demonstrated that TRH was critical for the basal activity and responsiveness to thyroid hormone of the TSHβ gene. cDNA microarray and K-means cluster analyses with pituitaries from wild-type mice, TRH-deficient mice and TRH-deficient mice with thyroid hormone replacement revealed that the largest and most consistent decrease in expression in the absence of TRH and on supplementation with thyroid hormone was shown by the TSHβ gene, and the NR4A1 gene belonged to the same cluster as and showed a similar expression profile to the TSHβ gene. Immunohistochemical analysis demonstrated that NR4A1 was expressed not only in ACTH- and FSH- producing cells but also in thyrotrophs and the expression was remarkably reduced in TRH-deficient pituitary. Furthermore, experiments in vitro demonstrated that incubation with TRH in GH4C1 cells increased the endogenous NR4A1 mRNA level by approximately 50-fold within one hour, and this stimulation was inhibited by inhibitors for PKC and ERK1/2. Western blot analysis confirmed that TRH increased NR4A1 expression within 2 h. A series of deletions of the promoter demonstrated that the region between bp -138 and +37 of the TSHβ gene was responsible for the TRH-induced stimulation, and Chip analysis revealed that NR4A1 was recruited to this region. Conversely, knockdown of NR4A1 by siRNA led to a significant reduction in TRH-induced TSHβ promoter activity. Furthermore, TRH stimulated NR4A1 promoter activity through the TRH receptor. These findings demonstrated that 1) TRH is a highly specific regulator of the TSHβ gene, and 2) TRH mediated induction of the TSHβ gene, at least in part by sequential stimulation of the NR4A1-TSHβ genes through a PKC and ERK1/2 pathway.
Conflict of interest statement
Figures




References
-
- Boler J, Enzmann F, Folkers K, Bowers C, Schally A. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem Biophys Res Commun. 1969;37:705–710. - PubMed
-
- Burgus R, Dunn T, Ward D, Vale W, Amoss M, et al. [Synthetic polypeptide derivatives with thyrotropin releasing factor hypophysiotropic activity]. C R Acad Sci Hebd Seances Acad Sci D. 1969;268:2116–2118. - PubMed
-
- Jackson I. Thyrotropin-releasing hormone. N Engl J Med. 1982;306:145–155. - PubMed
-
- Carr F, Shupnik M, Burnside J, Chin W. Thyrotropin-releasing hormone stimulates the activity of the rat thyrotropin beta-subunit gene promoter transfected into pituitary cells. Mol Endocrinol. 1989;3:717–724. - PubMed
-
- Carr F, Fisher C, Fein H, Smallridge R. Thyrotropin-releasing hormone stimulates c-jun and c-fos messenger ribonucleic acid levels: implications for calcium mobilization and protein kinase-C activation. Endocrinology. 1993;133:1700–1707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

