Defence signalling triggered by Flg22 and Harpin is integrated into a different stilbene output in Vitis cells
- PMID: 22792328
- PMCID: PMC3391249
- DOI: 10.1371/journal.pone.0040446
Defence signalling triggered by Flg22 and Harpin is integrated into a different stilbene output in Vitis cells
Abstract
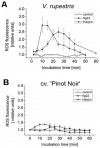
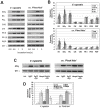
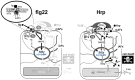
Plants can activate defence to pathogen attack by two layers of innate immunity: basal immunity triggered by pathogen-associated molecular pattern (PAMP) triggered immunity (PTI) and effector-triggered immunity (ETI) linked with programmed cell death. Flg22 and Harpin are evolutionary distinct bacterial PAMPs. We have previously shown that Harpin triggers hypersensitive cell death mimicking ETI in Vitis rupestris, but not in the Vitis vinifera cultivar 'Pinot Noir'. In contrast, the bacterial PAMP flg22 activating PTI does not trigger cell death. To get insight into the defence signalling triggered by flg22 and Harpin, we compared cellular responses upon flg22 and Harpin treatment in the two Vitis cell lines. We found that extracellular alkalinisation was blocked by inhibition of calcium influx, and modulated by pharmacological manipulation of the cytoskeleton and mitogen-activated protein kinase activity with quantitative differences between cell lines and type of PAMPs. In addition, an oxidative burst was detected that was much stronger and faster in response to Harpin as compared to flg22. In V. rupestris, both flg22 and Harpin induced transcripts of defence-related genes including stilbene synthase, microtubule disintegration and actin bundling in a similar way, whereas they differed in V. vinifera cv. 'Pinot Noir'. In contrast to Harpin, flg22 failed to trigger significant levels of the stilbene trans-resveratrol, and did not induce hypersensitive cell death even in the highly responsive V. rupestris. We discuss these data in a model, where flg22- and Harpin-triggered defence shares a part of early signal components, but differs in perception, oxidative burst, and integration into a qualitatively different stilbene output, such that for flg22 a basal PTI is elicited in both cell lines, while Harpin induces cell death mimicking an ETI-like pattern of defence.
Conflict of interest statement
Figures





References
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Cunnac S, Lindeberg M, Collmer A. Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr Opin Microbiol. 2009;12:53–60. - PubMed
-
- Tsuda K, Katagiri F. Comparing signalling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol. 2010;13:459–465. - PubMed
-
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

