Morphological and molecular diagnosis of anisakid nematode larvae from cutlassfish (Trichiurus lepturus) off the coast of Rio de Janeiro, Brazil
- PMID: 22792329
- PMCID: PMC3392247
- DOI: 10.1371/journal.pone.0040447
Morphological and molecular diagnosis of anisakid nematode larvae from cutlassfish (Trichiurus lepturus) off the coast of Rio de Janeiro, Brazil
Abstract
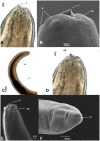
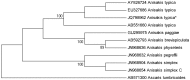
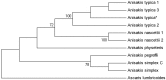
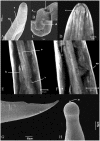
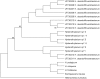
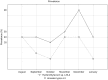
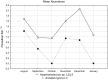
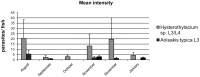
Anisakid nematode larvae from Trichiurus lepturus off coast of Rio de Janeiro were studied using light, laser confocal and scanning electron microscopy, in addition to a molecular approach. Mitochondrial cytochrome c-oxidase subunit 2 (mtDNA cox-2), partial 28S (LSU) and internal transcribed spacers (ITS-1, 5.8S, ITS-2) of ribosomal DNA were amplified using the polymerase chain reaction and sequenced to evaluate the phylogenetic relationships between the nematode taxa. The morphological and genetic profiles confirmed that, of the 1,030 larvae collected from the 64 fish examined, 398 were analysed, of which 361 were Hysterothylacium sp. and 37 were Anisakis typica. Larvae of Hysterothylacium sp. were not identified to the species level due to the absence of similar sequences for adult parasites; however, the ITS sequence clustered in the phylogenetic tree with sequences of H. deardorffoverstreetorum, whereas an mtDNA cox-2 and LSU concatenated phylogenetic analysis demonstrated the presence of two clades, both of them under the same name as the larval H. deardorffoverstreetorum. Data on the occurrence of parasites during the winter and summer months were compared using the t-test. The greatest prevalence and intensity of infection were recorded for larval Hysterothylacium, with a prevalence of 51.56% and an intensity of up to 55 parasites per fish. The larval Anisakis exhibit a higher abundance and intensity of infection in the winter months, and those of Hysterothylacium during the summer. However, the t-test indicated no significant differences between the abundance and intensity of infection recorded during the months of collection for either of these larval nematodes. All sequences generated in this study were deposited in GenBank.
Conflict of interest statement
Figures



References
-
- Mattiucci S, Nascetti G. Advances and trends in molecular systematics of anisakid nematodes, with implications for their evolutionary, ecology and host-parasite co-evolutionary processes. Rollinson D, Hay SI, editors. Academic Press. 2008;66:47–148. editors. Advances in Parasitology. - PubMed
-
- Klimpel S, Palm HW. Anisakid nematode (Ascaridoidea) life cycles and distribution. Increasing zoonotic potential in the time of climate change? Parasitology Research Monographs. 2001;2:201–222.
-
- Anderson RC. Nematode parasites of vertebrates: Their development and transmission. Wallingford: CABI Publishing. 672 p. 2000.
-
- Mattiucci S, Nascetti G, Cianchi R, Paggi L, Arduino P, et al. Genetic and ecological data on the Anisakis simplex complex with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). Journal of Parasitology. 1997;83:401–416. - PubMed
-
- Nadler SA, D’Amelio S, Dailey MD, Paggi L, Siu S, et al. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from northern Pacific marine mammals. Journal of Parasitology, 91. 2005;(6):1413–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

