SCaMC-1Like a member of the mitochondrial carrier (MC) family preferentially expressed in testis and localized in mitochondria and chromatoid body
- PMID: 22792342
- PMCID: PMC3391283
- DOI: 10.1371/journal.pone.0040470
SCaMC-1Like a member of the mitochondrial carrier (MC) family preferentially expressed in testis and localized in mitochondria and chromatoid body
Abstract
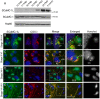
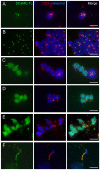
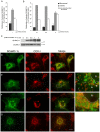
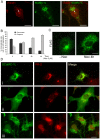
Mitochondrial carriers (MC) form a highly conserved family involved in solute transport across the inner mitochondrial membrane in eukaryotes. In mammals, ATP-Mg/Pi carriers, SCaMCs, form the most complex subgroup with four paralogs, SCaMC-1, -2, -3 and -3L, and several splicing variants. Here, we report the tissue distribution and subcellular localization of a mammalian-specific SCaMC paralog, 4930443G12Rik/SCaMC-1Like (SCaMC-1L), which displays unanticipated new features. SCaMC-1L proteins show higher amino acid substitution rates than its closest paralog SCaMC-1. In mouse, SCaMC-1L expression is restricted to male germ cells and regulated during spermatogenesis but unexpectedly its localization is not limited to mitochondrial structures. In mature spermatids SCaMC-1L is detected in the mitochondrial sheath but in previous differentiation stages appears associated to cytosolic granules which colocalize with specific markers of the chromatoid body (CB) in post-meiotic round spermatids and inter-mitochondrial cement (IMC) in spermatocytes. The origin of this atypical distribution was further investigated by transient expression in cell lines. Similarly to male germ cells, in addition to mitochondrial and cytosolic distribution, a fraction of SCaMC-1L-expressing COS-7 cells display cytosolic SCaMC-1L-aggregates which exhibit aggresomal-like features as the CB. Our results indicate that different regions of SCaMC-1L hinder its import into mitochondria and this apparently favours the formation of cytosolic aggregates in COS-7 cells. This mechanism could be also operational in male germ cells and explain the incorporation of SCaMC-1L into germinal granules.
Conflict of interest statement
Figures




References
-
- Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447:689–709. - PubMed
-
- Kunji ER. The role and structure of mitochondrial carriers. FEBS Lett. 2004;564:239–44. - PubMed
-
- Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, et al. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem. 2007;282:29658–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

