Novel scabies mite serpins inhibit the three pathways of the human complement system
- PMID: 22792350
- PMCID: PMC3394726
- DOI: 10.1371/journal.pone.0040489
Novel scabies mite serpins inhibit the three pathways of the human complement system
Abstract
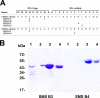
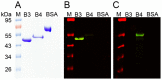
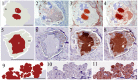
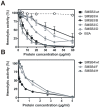
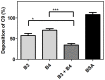
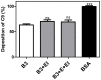
Scabies is a parasitic infestation of the skin by the mite Sarcoptes scabiei that causes significant morbidity worldwide, in particular within socially disadvantaged populations. In order to identify mechanisms that enable the scabies mite to evade human immune defenses, we have studied molecules associated with proteolytic systems in the mite, including two novel scabies mite serine protease inhibitors (SMSs) of the serpin superfamily. Immunohistochemical studies revealed that within mite-infected human skin SMSB4 (54 kDa) and SMSB3 (47 kDa) were both localized in the mite gut and feces. Recombinant purified SMSB3 and SMSB4 did not inhibit mite serine and cysteine proteases, but did inhibit mammalian serine proteases, such as chymotrypsin, albeit inefficiently. Detailed functional analysis revealed that both serpins interfered with all three pathways of the human complement system at different stages of their activation. SMSB4 inhibited mostly the initial and progressing steps of the cascades, while SMSB3 showed the strongest effects at the C9 level in the terminal pathway. Additive effects of both serpins were shown at the C9 level in the lectin pathway. Both SMSs were able to interfere with complement factors without protease function. A range of binding assays showed direct binding between SMSB4 and seven complement proteins (C1, properdin, MBL, C4, C3, C6 and C8), while significant binding of SMSB3 occurred exclusively to complement factors without protease function (C4, C3, C8). Direct binding was observed between SMSB4 and the complement proteases C1s and C1r. However no complex formation was observed between either mite serpin and the complement serine proteases C1r, C1s, MASP-1, MASP-2 and MASP-3. No catalytic inhibition by either serpin was observed for any of these enzymes. In summary, the SMSs were acting at several levels mediating overall inhibition of the complement system and thus we propose that they may protect scabies mites from complement-mediated gut damage.
Conflict of interest statement
Figures


Similar articles
-
A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth.PLoS Negl Trop Dis. 2014 Jun 19;8(6):e2928. doi: 10.1371/journal.pntd.0002928. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24945501 Free PMC article.
-
Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway.PLoS Negl Trop Dis. 2014 May 22;8(5):e2872. doi: 10.1371/journal.pntd.0002872. eCollection 2014 May. PLoS Negl Trop Dis. 2014. PMID: 24854034 Free PMC article.
-
Scabies mite inactivated serine protease paralogs inhibit the human complement system.J Immunol. 2009 Jun 15;182(12):7809-17. doi: 10.4049/jimmunol.0804205. J Immunol. 2009. PMID: 19494305
-
Parasitic scabies mites and associated bacteria joining forces against host complement defence.Parasite Immunol. 2014 Nov;36(11):585-93. doi: 10.1111/pim.12133. Parasite Immunol. 2014. PMID: 25081184 Review.
-
Host immune responses to the itch mite, Sarcoptes scabiei, in humans.Parasit Vectors. 2017 Aug 10;10(1):385. doi: 10.1186/s13071-017-2320-4. Parasit Vectors. 2017. PMID: 28797273 Free PMC article. Review.
Cited by
-
Gene silencing by RNA interference in Sarcoptes scabiei: a molecular tool to identify novel therapeutic targets.Parasit Vectors. 2017 Jun 10;10(1):289. doi: 10.1186/s13071-017-2226-1. Parasit Vectors. 2017. PMID: 28601087 Free PMC article.
-
Rhipicephalus microplus serine protease inhibitor family: annotation, expression and functional characterisation assessment.Parasit Vectors. 2015 Jan 7;8:7. doi: 10.1186/s13071-014-0605-4. Parasit Vectors. 2015. PMID: 25564202 Free PMC article.
-
High-quality nuclear genome for Sarcoptes scabiei-A critical resource for a neglected parasite.PLoS Negl Trop Dis. 2020 Oct 1;14(10):e0008720. doi: 10.1371/journal.pntd.0008720. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33001992 Free PMC article.
-
Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics.Toxins (Basel). 2019 Jun 19;11(6):355. doi: 10.3390/toxins11060355. Toxins (Basel). 2019. PMID: 31248109 Free PMC article. Review.
-
Phylogenetic relationships, stage-specific expression and localisation of a unique family of inactive cysteine proteases in Sarcoptes scabiei.Parasit Vectors. 2018 May 16;11(1):301. doi: 10.1186/s13071-018-2862-0. Parasit Vectors. 2018. PMID: 29769145 Free PMC article.
References
-
- Hengge UR, Currie BJ, Jäger G, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. The Lancet Infectious Diseases. 2006;6:769–779. - PubMed
-
- McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis. 2004;4:240–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

