In vitro assays using primary embryonic mouse lymphatic endothelial cells uncover key roles for FGFR1 signalling in lymphangiogenesis
- PMID: 22792354
- PMCID: PMC3391274
- DOI: 10.1371/journal.pone.0040497
In vitro assays using primary embryonic mouse lymphatic endothelial cells uncover key roles for FGFR1 signalling in lymphangiogenesis
Abstract
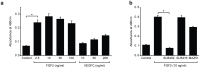
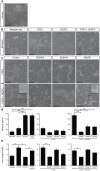
Despite the importance of blood vessels and lymphatic vessels during development and disease, the signalling pathways underpinning vessel construction remain poorly characterised. Primary mouse endothelial cells have traditionally proven difficult to culture and as a consequence, few assays have been developed to dissect gene function and signal transduction pathways in these cells ex vivo. Having established methodology for the purification, short-term culture and transfection of primary blood (BEC) and lymphatic (LEC) vascular endothelial cells isolated from embryonic mouse skin, we sought to optimise robust assays able to measure embryonic LEC proliferation, migration and three-dimensional tube forming ability in vitro. In the course of developing these assays using the pro-lymphangiogenic growth factors FGF2 and VEGF-C, we identified previously unrecognised roles for FGFR1 signalling in lymphangiogenesis. The small molecule FGF receptor tyrosine kinase inhibitor SU5402, but not inhibitors of VEGFR-2 (SU5416) or VEGFR-3 (MAZ51), inhibited FGF2 mediated LEC proliferation, demonstrating that FGF2 promotes proliferation directly via FGF receptors and independently of VEGF receptors in primary embryonic LEC. Further investigation revealed that FGFR1 was by far the predominant FGF receptor expressed by primary embryonic LEC and correspondingly, siRNA-mediated FGFR1 knockdown abrogated FGF2 mediated LEC proliferation. While FGF2 potently promoted LEC proliferation and migration, three dimensional tube formation assays revealed that VEGF-C primarily promoted LEC sprouting and elongation, illustrating that FGF2 and VEGF-C play distinct, cooperative roles in lymphatic vascular morphogenesis. These assays therefore provide useful tools able to dissect gene function in cellular events important for lymphangiogenesis and implicate FGFR1 as a key player in developmental lymphangiogenesis in vivo.
Conflict of interest statement
Figures

Similar articles
-
The β1-integrin plays a key role in LEC invasion in an optimized 3-D collagen matrix model.Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C1045-C1058. doi: 10.1152/ajpcell.00299.2020. Epub 2020 Oct 14. Am J Physiol Cell Physiol. 2020. PMID: 33052069
-
Modulation of VEGF-induced migration and network formation by lymphatic endothelial cells: Roles of platelets and podoplanin.Platelets. 2018 Jul;29(5):486-495. doi: 10.1080/09537104.2017.1336210. Epub 2017 Jul 20. Platelets. 2018. PMID: 28727496 Free PMC article.
-
Vascular endothelial growth factor-C secreted by pancreatic cancer cell line promotes lymphatic endothelial cell migration in an in vitro model of tumor lymphangiogenesis.Pancreas. 2007 May;34(4):444-51. doi: 10.1097/mpa.0b13e31803dd307. Pancreas. 2007. PMID: 17446844
-
Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer.Cancer Metastasis Rev. 2018 Sep;37(2-3):369-384. doi: 10.1007/s10555-018-9734-0. Cancer Metastasis Rev. 2018. PMID: 29858743 Review.
-
Lymphangiogenic growth factors, receptors and therapies.Thromb Haemost. 2003 Aug;90(2):167-84. doi: 10.1160/TH03-04-0200. Thromb Haemost. 2003. PMID: 12888864 Review.
Cited by
-
Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma.J Hepatol. 2019 Apr;70(4):700-709. doi: 10.1016/j.jhep.2018.12.004. Epub 2018 Dec 14. J Hepatol. 2019. PMID: 30553841 Free PMC article.
-
Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer's disease.Neural Regen Res. 2018 Apr;13(4):709-716. doi: 10.4103/1673-5374.230299. Neural Regen Res. 2018. PMID: 29722325 Free PMC article.
-
FGFs: crucial factors that regulate tumour initiation and progression.Cell Prolif. 2016 Aug;49(4):438-47. doi: 10.1111/cpr.12275. Epub 2016 Jul 7. Cell Prolif. 2016. PMID: 27383016 Free PMC article. Review.
-
VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model.Microcirculation. 2014 Aug;21(6):532-40. doi: 10.1111/micc.12132. Microcirculation. 2014. PMID: 24654984 Free PMC article.
-
Lymphatic Drainage-Promoting Effects by Engraftment of Artificial Lymphatic Vascular Tissue Based on Human Adipose Tissue-Derived Mesenchymal Stromal Cells in Mice.J Tissue Eng Regen Med. 2023 Nov 6;2023:7626767. doi: 10.1155/2023/7626767. eCollection 2023. J Tissue Eng Regen Med. 2023. PMID: 40226423 Free PMC article.
References
-
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. - PubMed
-
- Johnston MG, Walker MA. Lymphatic endothelial and smooth-muscle cells in tissue culture. In Vitro. 1984;20:566–572. - PubMed
-
- Pepper MS, Wasi S, Ferrara N, Orci L, Montesano R. In vitro angiogenic and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res. 1994;210:298–305. - PubMed
-
- Gnepp DR, Chandler W. Tissue culture of human and canine thoracic duct endothelium. In Vitro Cell Dev Biol. 1985;21:200–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

