Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening
- PMID: 22792356
- PMCID: PMC3392228
- DOI: 10.1371/journal.pone.0040501
Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening
Abstract
Background: Loss of function mutations in the DJ-1 gene have been linked to recessively inherited forms of Parkinsonism. Mitochondrial dysfunction and increased oxidative stress are thought to be key events in the pathogenesis of Parkinson's disease. Although it has been reported that DJ-1 serves as scavenger for reactive oxidative species (ROS) by oxidation on its cysteine residues, how loss of DJ-1 affects mitochondrial function is less clear.
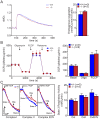
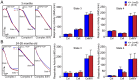
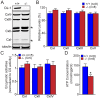
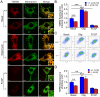
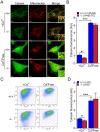
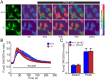
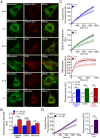
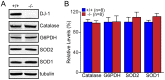
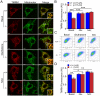
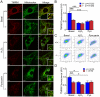
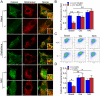
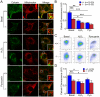
Methodology/principal findings: Using primary mouse embryonic fibroblasts (MEFs) or brains from DJ-1-/- mice, we found that loss of DJ-1 does not affect mitochondrial respiration. Specifically, endogenous respiratory activity as well as basal and maximal respiration are normal in intact DJ-1-/- MEFs, and substrate-specific state 3 and state 4 mitochondrial respiration are also unaffected in permeabilized DJ-1-/- MEFs and in isolated mitochondria from the cerebral cortex of DJ-1-/- mice at 3 months or 2 years of age. Expression levels and activities of all individual complexes composing the electron transport system are unchanged, but ATP production is reduced in DJ-1-/- MEFs. Mitochondrial transmembrane potential is decreased in the absence of DJ-1. Furthermore, mitochondrial permeability transition pore opening is increased, whereas mitochondrial calcium levels are unchanged in DJ-1-/- cells. Consistent with earlier reports, production of reactive oxygen species (ROS) is increased, though levels of antioxidative enzymes are unaltered. Interestingly, the decreased mitochondrial transmembrane potential and the increased mitochondrial permeability transition pore opening in DJ-1-/- MEFs can be restored by antioxidant treatment, whereas oxidative stress inducers have the opposite effects on mitochondrial transmembrane potential and mitochondrial permeability transition pore opening.
Conclusions/significance: Our study shows that loss of DJ-1 does not affect mitochondrial respiration or mitochondrial calcium levels but increases ROS production, leading to elevated mitochondrial permeability transition pore opening and reduced mitochondrial transmembrane potential.
Conflict of interest statement
Figures
Similar articles
-
Brain mitochondria from DJ-1 knockout mice show increased respiration-dependent hydrogen peroxide consumption.Redox Biol. 2014 Apr 24;2:667-72. doi: 10.1016/j.redox.2014.04.010. eCollection 2014. Redox Biol. 2014. PMID: 24936441 Free PMC article.
-
DJ-1 protects against cell death following acute cardiac ischemia-reperfusion injury.Cell Death Dis. 2014 Feb 27;5(2):e1082. doi: 10.1038/cddis.2014.41. Cell Death Dis. 2014. PMID: 24577080 Free PMC article.
-
Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1.PLoS One. 2010 Feb 23;5(2):e9367. doi: 10.1371/journal.pone.0009367. PLoS One. 2010. PMID: 20186336 Free PMC article.
-
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release.Physiol Rev. 2014 Jul;94(3):909-50. doi: 10.1152/physrev.00026.2013. Physiol Rev. 2014. PMID: 24987008 Free PMC article. Review.
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
Cited by
-
Gender-dimorphic regulation of DJ1 and its interactions with metabolic proteins in streptozotocin-induced diabetic rats.J Cell Mol Med. 2015 May;19(5):996-1009. doi: 10.1111/jcmm.12490. Epub 2015 Feb 27. J Cell Mol Med. 2015. PMID: 25726699 Free PMC article.
-
Oxidative stress and Parkinson's disease.Front Neuroanat. 2015 Jul 8;9:91. doi: 10.3389/fnana.2015.00091. eCollection 2015. Front Neuroanat. 2015. PMID: 26217195 Free PMC article. Review.
-
On the Chemical and Biological Characteristics of Multifunctional Compounds for the Treatment of Parkinson's Disease.Antioxidants (Basel). 2023 Jan 17;12(2):214. doi: 10.3390/antiox12020214. Antioxidants (Basel). 2023. PMID: 36829773 Free PMC article. Review.
-
Natural Molecules From Chinese Herbs Protecting Against Parkinson's Disease via Anti-oxidative Stress.Front Aging Neurosci. 2018 Aug 28;10:246. doi: 10.3389/fnagi.2018.00246. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30233351 Free PMC article.
-
mPTP opening caused by Cdk5 loss is due to increased mitochondrial Ca2+ uptake.Oncogene. 2020 Mar;39(13):2797-2806. doi: 10.1038/s41388-020-1188-5. Epub 2020 Feb 5. Oncogene. 2020. PMID: 32024968 Free PMC article.
References
-
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, et al. Mutations in th parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. - PubMed
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
-
- Shen J, Cookson MR. Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron. 2004;43:301–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

