Hilar GABAergic interneuron activity controls spatial learning and memory retrieval
- PMID: 22792368
- PMCID: PMC3390383
- DOI: 10.1371/journal.pone.0040555
Hilar GABAergic interneuron activity controls spatial learning and memory retrieval
Abstract
Background: Although extensive research has demonstrated the importance of excitatory granule neurons in the dentate gyrus of the hippocampus in normal learning and memory and in the pathogenesis of amnesia in Alzheimer's disease (AD), the role of hilar GABAergic inhibitory interneurons, which control the granule neuron activity, remains unclear.
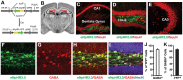
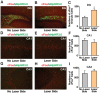
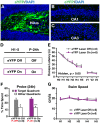
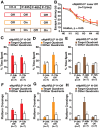
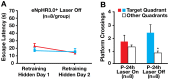
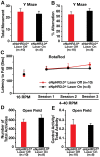
Methodology and principal findings: We explored the function of hilar GABAergic interneurons in spatial learning and memory by inhibiting their activity through Cre-dependent viral expression of enhanced halorhodopsin (eNpHR3.0)--a light-driven chloride pump. Hilar GABAergic interneuron-specific expression of eNpHR3.0 was achieved by bilaterally injecting adeno-associated virus containing a double-floxed inverted open-reading frame encoding eNpHR3.0 into the hilus of the dentate gyrus of mice expressing Cre recombinase under the control of an enhancer specific for GABAergic interneurons. In vitro and in vivo illumination with a yellow laser elicited inhibition of hilar GABAergic interneurons and consequent activation of dentate granule neurons, without affecting pyramidal neurons in the CA3 and CA1 regions of the hippocampus. We found that optogenetic inhibition of hilar GABAergic interneuron activity impaired spatial learning and memory retrieval, without affecting memory retention, as determined in the Morris water maze test. Importantly, optogenetic inhibition of hilar GABAergic interneuron activity did not alter short-term working memory, motor coordination, or exploratory activity.
Conclusions and significance: Our findings establish a critical role for hilar GABAergic interneuron activity in controlling spatial learning and memory retrieval and provide evidence for the potential contribution of GABAergic interneuron impairment to the pathogenesis of amnesia in AD.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

