Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury
- PMID: 22792383
- PMCID: PMC3394706
- DOI: 10.1371/journal.pone.0040602
Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury
Abstract
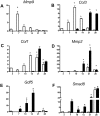
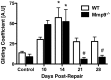
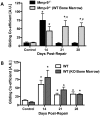
The pathogenesis of adhesions following primary tendon repair is poorly understood, but is thought to involve dysregulation of matrix metalloproteinases (Mmps). We have previously demonstrated that Mmp9 gene expression is increased during the inflammatory phase following murine flexor digitorum (FDL) tendon repair in association with increased adhesions. To further investigate the role of Mmp9, the cellular, molecular, and biomechanical features of healing were examined in WT and Mmp9(-/-) mice using the FDL tendon repair model. Adhesions persisted in WT, but were reduced in Mmp9(-/-) mice by 21 days without any decrease in strength. Deletion of Mmp9 resulted in accelerated expression of neo-tendon associated genes, Gdf5 and Smad8, and delayed expression of collagen I and collagen III. Furthermore, WT bone marrow cells (GFP(+)) migrated specifically to the tendon repair site. Transplanting myeloablated Mmp9(-/-) mice with WT marrow cells resulted in greater adhesions than observed in Mmp9(-/-) mice and similar to those seen in WT mice. These studies show that Mmp9 is primarily derived from bone marrow cells that migrate to the repair site, and mediates adhesion formation in injured tendons. Mmp9 is a potential target to limit adhesion formation in tendon healing.
Conflict of interest statement
Figures



References
-
- Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am. 2003;85-A:539–550. - PubMed
-
- Lin T. Biomechanics of tendon inury and repair. Journal of Biomechanics. 2004;37:865–877. - PubMed
-
- Gelberman RH, Botte MJ, Spiegelman JJ, Akeson WH. The excursion and deformation of repaired flexor tendons treated with protected early motion. J Hand Surg [Am] 1986;11:106–110. - PubMed
-
- Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am] 1982;7:170–175. - PubMed
-
- Sharma P, Maffulli N. Basic biology of tendon injury and healing. Surgeon. 2005;3:309–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

