Sensitivity profile for orientation selectivity in the visual cortex of goggle-reared mice
- PMID: 22792390
- PMCID: PMC3391291
- DOI: 10.1371/journal.pone.0040630
Sensitivity profile for orientation selectivity in the visual cortex of goggle-reared mice
Abstract
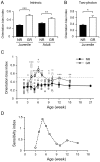
It has been widely accepted that ocular dominance in the responses of visual cortical neurons can change depending on visual experience in a postnatal period. However, experience-dependent plasticity for orientation selectivity, which is another important response property of visual cortical neurons, is not yet fully understood. To address this issue, using intrinsic signal imaging and two-photon calcium imaging we attempted to observe the alteration of orientation selectivity in the visual cortex of juvenile and adult mice reared with head-mounted goggles, through which animals can experience only the vertical orientation. After one week of goggle rearing, the density of neurons optimally responding to the exposed orientation increased, while that responding to unexposed orientations decreased. These changes can be interpreted as a reallocation of preferred orientations among visually responsive neurons. Our obtained sensitivity profile for orientation selectivity showed a marked peak at 5 weeks and sustained elevation at 12 weeks and later. These features indicate the existence of a critical period between 4 and 7 weeks and residual orientation plasticity in adult mice. The presence of a dip in the sensitivity profile at 10 weeks suggests that different mechanisms are involved in orientation plasticity in childhood and adulthood.
Conflict of interest statement
Figures
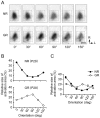

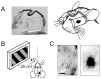
 ) and 5 s. (C) Color-coded images of vigorously responsive neurons in single slices of an NR mouse (left) and a mouse GR from P23 to P31 (right), which were reconstructed from two-photon Ca2+ imaging conducted at P42 and P31, respectively. The left image was reconstructed from the Ca2+ signals in the slice shown in (A). The color of the dots indicates the preferred orientation, whereas gray dots indicate responsive but unoriented neurons. The color code is shown below the left image. (D) Group-averaged orientation distributions for NR (n = 3) and GR (n = 3) mice. Error bars indicate SE. The scale bar indicates 100 µm.
) and 5 s. (C) Color-coded images of vigorously responsive neurons in single slices of an NR mouse (left) and a mouse GR from P23 to P31 (right), which were reconstructed from two-photon Ca2+ imaging conducted at P42 and P31, respectively. The left image was reconstructed from the Ca2+ signals in the slice shown in (A). The color of the dots indicates the preferred orientation, whereas gray dots indicate responsive but unoriented neurons. The color code is shown below the left image. (D) Group-averaged orientation distributions for NR (n = 3) and GR (n = 3) mice. Error bars indicate SE. The scale bar indicates 100 µm.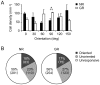
References
-
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. - PubMed
-
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. - PubMed
-
- Dräger UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978;41:28–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

