Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses
- PMID: 22792401
- PMCID: PMC3394713
- DOI: 10.1371/journal.pone.0040733
Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses
Abstract
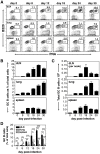
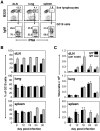
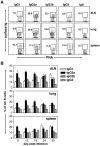
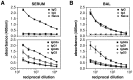
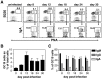
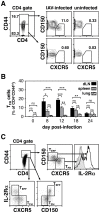
Protection from influenza A virus (IAV) challenge requires switched, high affinity Abs derived from long-lived memory B cells and plasma cells. These B cell subsets are generated in germinal centers (GCs), hallmark structures of T helper cell-driven B cell immunity. A full understanding of the GC reaction after respiratory IAV infection is lacking, as is the characterization of T follicular helper (T(FH)) cells that support GCs. Here, GC B cell and T(FH) cell responses were studied in mice following pulmonary challenge with IAV. Marked GC reactions were induced in draining lymph nodes (dLNs), lung, spleen and nasal-associated lymphoid tissue (NALT), although the magnitude and kinetics of the response was site-specific. Examination of switching within GCs demonstrated IgG2(+) cells to compose the largest fraction in dLNs, lung and spleen. IgA(+) GC B cells were infrequent in these sites, but composed a significant subset of the switched GC population in NALT. Further experiments demonstrated splenectomized mice to withstand a lethal recall challenge, suggesting the spleen to be unnecessary for long-term protection in spite of strong GC responses in this organ. Final studies showed that T(FH) cell numbers were highest in dLNs and spleen, and peaked in all sites prior to the height of the GC reaction. T(FH) cells purified from dLNs generated IL-21 and IFNγ upon activation, although CD4(+)CXCR5(-) T effector cells produced higher levels of all cytokines. Collectively, these findings reveal respiratory IAV infection to induce strong T helper cell-driven B cell responses in various organs, with each site displaying unique attributes.
Conflict of interest statement
Figures


Similar articles
-
Single and coexpression of CXCR4 and CXCR5 identifies CD4 T helper cells in distinct lymph node niches during influenza virus infection.J Virol. 2012 Jul;86(13):7146-57. doi: 10.1128/JVI.06904-11. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532671 Free PMC article.
-
Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article.
-
Primary and long-term B-cell responses in the upper airway and lung after influenza A virus infection.Immunol Res. 2014 Aug;59(1-3):73-80. doi: 10.1007/s12026-014-8541-0. Immunol Res. 2014. PMID: 24838149 Review.
-
Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig.J Immunol. 1996 Oct 1;157(7):2947-52. J Immunol. 1996. PMID: 8816401
-
T Cell Subsets in the Germinal Center: Lessons from the Macaque Model.Front Immunol. 2018 Feb 26;9:348. doi: 10.3389/fimmu.2018.00348. eCollection 2018. Front Immunol. 2018. PMID: 29535724 Free PMC article. Review.
Cited by
-
Sublingual Vaccination with Live Influenza Virus Induces Better Protection Than Oral Immunization in Mice.Life (Basel). 2022 Jun 29;12(7):975. doi: 10.3390/life12070975. Life (Basel). 2022. PMID: 35888065 Free PMC article.
-
The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms.Mucosal Immunol. 2014 May;7(3):455-66. doi: 10.1038/mi.2014.11. Epub 2014 Feb 26. Mucosal Immunol. 2014. PMID: 24569801 Review.
-
Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus.J Virol. 2014 Oct;88(20):11995-2005. doi: 10.1128/JVI.01833-14. Epub 2014 Aug 6. J Virol. 2014. PMID: 25100838 Free PMC article.
-
SARS-CoV-2-specific circulating T follicular helper cells correlate with neutralizing antibodies and increase during early convalescence.PLoS Pathog. 2021 Jul 16;17(7):e1009761. doi: 10.1371/journal.ppat.1009761. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34270631 Free PMC article.
-
Prior infection with influenza virus but not vaccination leaves a long-term immunological imprint that intensifies the protective efficacy of antigenically drifted vaccine strains.Vaccine. 2016 Jan 20;34(4):495-502. doi: 10.1016/j.vaccine.2015.11.077. Epub 2015 Dec 17. Vaccine. 2016. PMID: 26706277 Free PMC article.
References
-
- Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. - PubMed
-
- Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. - PubMed
-
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

