Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control study
- PMID: 22792416
- PMCID: PMC3388586
- DOI: 10.1155/2012/636419
Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control study
Abstract
Problem: There was no direct correlation between plasma and placental oxidative damage parameters and inflammation and evidence of TLR4 pathway activation in the placenta in preeclamptic (PE) patients.
Method of study: 33 PE patients and 33 normotensive pregnant women were included. The maternal section of the placenta and blood were collected to the determination of oxidative damage markers (thiobarbituric acid reactive species and protein carbonyls), inflammatory response (interleukin-6 and myeloperoxidase activity), and activation of the TLR-4-NF-kB pathway.
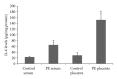
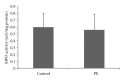
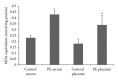
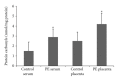
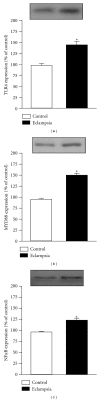
Results: An increase of IL-6 levels in both plasma and placenta was observed, but myeloperoxidase activity was not significantly different comparing the groups. Oxidative damage parameters were increased in plasma and placenta in PE patients. A significant increase of the protein levels of TLR-4 and NF-kB was observed in the placenta.
Conclusion: The TLR4-NF-kB pathway is upregulated in PE, probably generating local and systemic inflammatory response that is followed by local and systemic oxidative damage.
Figures
Similar articles
-
Positive Correlation between Enhanced Expression of TLR4/MyD88/NF-κB with Insulin Resistance in Placentae of Gestational Diabetes Mellitus.PLoS One. 2016 Jun 24;11(6):e0157185. doi: 10.1371/journal.pone.0157185. eCollection 2016. PLoS One. 2016. PMID: 27340831 Free PMC article.
-
Progesterone and vitamin D downregulate the activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-κB pathway in monocytes from pregnant women with preeclampsia.J Reprod Immunol. 2021 Apr;144:103286. doi: 10.1016/j.jri.2021.103286. Epub 2021 Feb 5. J Reprod Immunol. 2021. PMID: 33578174
-
Placental inflammation by HMGB1 activation of TLR4 at the syncytium.Placenta. 2018 Dec;72-73:53-61. doi: 10.1016/j.placenta.2018.10.011. Epub 2018 Nov 2. Placenta. 2018. PMID: 30501882
-
Plasma sFlt-1-to-PlGF ratio is correlated with inflammatory but not with oxidative stress in Chinese preeclamptic women.Arch Gynecol Obstet. 2009 Jul;280(1):91-7. doi: 10.1007/s00404-008-0874-2. Epub 2008 Dec 20. Arch Gynecol Obstet. 2009. PMID: 19099313
-
Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia.Oxid Med Cell Longev. 2019 Nov 4;2019:8238727. doi: 10.1155/2019/8238727. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781353 Free PMC article. Review.
Cited by
-
Analysis of the evolution of placental oxidative stress research from a bibliometric perspective.Front Pharmacol. 2024 Oct 17;15:1475244. doi: 10.3389/fphar.2024.1475244. eCollection 2024. Front Pharmacol. 2024. PMID: 39484166 Free PMC article.
-
Characterisation of cardiac health in the reduced uterine perfusion pressure model and a 3D cardiac spheroid model, of preeclampsia.Biol Sex Differ. 2021 Apr 20;12(1):31. doi: 10.1186/s13293-021-00376-1. Biol Sex Differ. 2021. PMID: 33879252 Free PMC article.
-
Four Pathways Involving Innate Immunity in the Pathogenesis of Preeclampsia.Front Cardiovasc Med. 2015 Apr 28;2:20. doi: 10.3389/fcvm.2015.00020. eCollection 2015. Front Cardiovasc Med. 2015. PMID: 26664892 Free PMC article.
-
Reduced Intellectual Ability in Offspring Born from Preeclamptic Mothers: A Prospective Cohort Study.Risk Manag Healthc Policy. 2020 Oct 8;13:2037-2046. doi: 10.2147/RMHP.S277521. eCollection 2020. Risk Manag Healthc Policy. 2020. PMID: 33116984 Free PMC article.
-
LPS Induces Preeclampsia-Like Phenotype in Rats and HTR8/SVneo Cells Dysfunction Through TLR4/p38 MAPK Pathway.Front Physiol. 2019 Aug 27;10:1030. doi: 10.3389/fphys.2019.01030. eCollection 2019. Front Physiol. 2019. PMID: 31507429 Free PMC article.
References
-
- Friedman SA, Taylor RN, Roberts JM. Pathophysiology of preeclampsia. Clinics in Perinatology. 1991;18(4):661–682. - PubMed
-
- Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33(1 I):83–89. - PubMed
-
- Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. Journal of Obstetrics and Gynaecology Research. 2008;34(6):948–951. - PubMed
-
- Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta. 2005;26(1):53–58. - PubMed
-
- Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. American Journal of Obstetrics and Gynecology. 1992;167(4 I):946–949. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

