Interactions between Leishmania braziliensis and Macrophages Are Dependent on the Cytoskeleton and Myosin Va
- PMID: 22792440
- PMCID: PMC3391898
- DOI: 10.1155/2012/275436
Interactions between Leishmania braziliensis and Macrophages Are Dependent on the Cytoskeleton and Myosin Va
Abstract
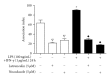
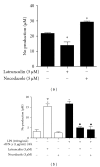
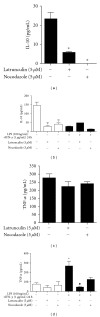
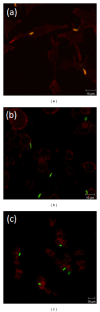
Leishmaniasis is a neglected tropical disease with no effective vaccines. Actin, microtubules and the actin-based molecular motor myosin Va were investigated for their involvement in Leishmania braziliensis macrophage interactions. Results showed a decrease in the association index when macrophages were without F-actin or microtubules regardless of the activation state of the macrophage. In the absence of F-actin, the production of NO in non-activated cells increased, while in activated cells, the production of NO was reduced independent of parasites. The opposite effect of an increased NO production was observed in the absence of microtubules. In activated cells, the loss of cytoskeletal components inhibited the release of IL-10 during parasite interactions. The production of IL-10 also decreased in the absence of actin or microtubules in non-activated macrophages. Only the disruption of actin altered the production of TNF-α in activated macrophages. The expression of myosin Va tail resulted in an acute decrease in the association index between transfected macrophages and L. braziliensis promastigotes. These data reveal the importance of F-actin, microtubules, and myosin-Va suggesting that modulation of the cytoskeleton may be a mechanism used by L. braziliensis to overcome the natural responses of macrophages to establish infections.
Figures
Similar articles
-
Involvement of lipids from Leishmania braziliensis promastigotes and amastigotes in macrophage activation.Mol Immunol. 2020 Sep;125:104-114. doi: 10.1016/j.molimm.2020.06.023. Epub 2020 Jul 10. Mol Immunol. 2020. PMID: 32659595
-
Leishmania (Viannia) braziliensis amastigotes induces the expression of TNFα and IL-10 by human peripheral blood mononuclear cells in vitro in a TLR4-dependent manner.Cytokine. 2016 Dec;88:184-192. doi: 10.1016/j.cyto.2016.09.009. Epub 2016 Sep 17. Cytokine. 2016. PMID: 27649507
-
Histone deacetylases inhibitors as new potential drugs against Leishmania braziliensis, the main causative agent of new world tegumentary leishmaniasis.Biochem Pharmacol. 2020 Oct;180:114191. doi: 10.1016/j.bcp.2020.114191. Epub 2020 Aug 7. Biochem Pharmacol. 2020. PMID: 32777278
-
Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility.Mol Biol Cell. 2001 Sep;12(9):2742-55. doi: 10.1091/mbc.12.9.2742. Mol Biol Cell. 2001. PMID: 11553713 Free PMC article.
-
Protection and Pathology in Leishmania braziliensis Infection.Pathogens. 2022 Apr 14;11(4):466. doi: 10.3390/pathogens11040466. Pathogens. 2022. PMID: 35456141 Free PMC article. Review.
Cited by
-
Gallic and ellagic acids: two natural immunomodulator compounds solve infection of macrophages by Leishmania major.Naunyn Schmiedebergs Arch Pharmacol. 2017 Sep;390(9):893-903. doi: 10.1007/s00210-017-1387-y. Epub 2017 Jun 22. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28643086
-
Assessment of knowledge, attitude, and practices towards canine visceral leishmaniasis based on the one health concept in Weliso and Ejaji Towns, Oromia, Ethiopia.Sci Rep. 2023 Nov 25;13(1):20765. doi: 10.1038/s41598-023-47340-0. Sci Rep. 2023. PMID: 38007526 Free PMC article.
-
CD100/Sema4D Increases Macrophage Infection by Leishmania (Leishmania) amazonensis in a CD72 Dependent Manner.Front Microbiol. 2018 Jun 5;9:1177. doi: 10.3389/fmicb.2018.01177. eCollection 2018. Front Microbiol. 2018. PMID: 29922261 Free PMC article.
-
High seroprevalence of Leishmania infantum infection in dogs and its associated risk factors in selected towns of Southwest and West Shewa zones of Oromia, Ethiopia.Vet Med Sci. 2022 Nov;8(6):2319-2328. doi: 10.1002/vms3.917. Epub 2022 Aug 30. Vet Med Sci. 2022. PMID: 36040358 Free PMC article.
-
Proteins Selected in Leishmania (Viannia) braziliensis by an Immunoproteomic Approach with Potential Serodiagnosis Applications for Tegumentary Leishmaniasis.Clin Vaccine Immunol. 2015 Nov;22(11):1187-96. doi: 10.1128/CVI.00465-15. Epub 2015 Sep 16. Clin Vaccine Immunol. 2015. PMID: 26376929 Free PMC article.
References
-
- Brelaz MC, de Oliveira AP, de Almeida AF, de Assis Souza M, de Brito ME, Pereira VR. Antigenic fractions of Leishmania (Viannia) braziliensis: the immune response characterization of patients at the initial phase of disease. Parasite Immunology. 2012;34(4):183–239. - PubMed
-
- Maüel J. Intracellular survival of protozoan parasites with special reference to Leishmania spp., Toxoplasma gondii and Trypanosoma cruzi . Advances in Parasitology. 1996;38:1–51. - PubMed
LinkOut - more resources
Full Text Sources

