Rhodium-catalyzed carbonylation of cyclopropyl substituted propargyl esters: a tandem 1,3-acyloxy migration [5 + 1] cycloaddition
- PMID: 22793991
- PMCID: PMC3420071
- DOI: 10.1021/jo300973r
Rhodium-catalyzed carbonylation of cyclopropyl substituted propargyl esters: a tandem 1,3-acyloxy migration [5 + 1] cycloaddition
Abstract
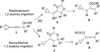
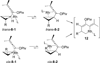
We have developed two different types of tandem reactions for the synthesis of highly functionalized cyclohexenones from cyclopropyl substituted propargyl esters. Both reactions were initiated by rhodium-catalyzed Saucy-Marbet 1,3-acyloxy migration. The resulting cyclopropyl substituted allenes derived from acyloxy migration then underwent [5 + 1] cycloaddition with CO. The acyloxy group not only eased the access to allene intermediates but also provided a handle for further selective functionalizations.
Figures

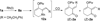


Similar articles
-
Rhodium-catalyzed carbonylation of 3-acyloxy-1,4-enynes for the synthesis of cyclopentenones.Org Lett. 2012 Mar 16;14(6):1584-7. doi: 10.1021/ol300330t. Epub 2012 Mar 2. Org Lett. 2012. PMID: 22381143 Free PMC article.
-
A computationally designed Rh(I)-catalyzed two-component [5+2+1] cycloaddition of ene-vinylcyclopropanes and CO for the synthesis of cyclooctenones.J Am Chem Soc. 2007 Aug 22;129(33):10060-1. doi: 10.1021/ja072505w. Epub 2007 Jul 27. J Am Chem Soc. 2007. PMID: 17655302 No abstract available.
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
-
Effect of ester on rhodium-catalyzed intermolecular [5+2] cycloaddition of 3-acyloxy-1,4-enynes and alkynes.Chem Commun (Camb). 2013 Apr 4;49(26):2616-8. doi: 10.1039/c3cc40634b. Chem Commun (Camb). 2013. PMID: 23435501 Free PMC article.
-
Rhodium-catalyzed acyloxy migration of propargylic esters in cycloadditions, inspiration from the recent "gold rush".Chem Soc Rev. 2012 Dec 7;41(23):7698-711. doi: 10.1039/c2cs35235d. Chem Soc Rev. 2012. PMID: 22895533 Free PMC article. Review.
Cited by
-
Rhodium-catalyzed tandem annulation and (5 + 1) cycloaddition: 3-hydroxy-1,4-enyne as the 5-carbon component.J Am Chem Soc. 2013 Nov 13;135(45):16797-800. doi: 10.1021/ja408829y. Epub 2013 Oct 31. J Am Chem Soc. 2013. PMID: 24164088 Free PMC article.
-
Au-Catalyzed Hexannulation and Pt-Catalyzed Pentannulation of Propargylic Ester Bearing a 2-Alkynyl-phenyl Substituent: A Comparative DFT Study.ACS Omega. 2018 Jan 29;3(1):1159-1169. doi: 10.1021/acsomega.7b01889. eCollection 2018 Jan 31. ACS Omega. 2018. PMID: 31457958 Free PMC article.
-
Mechanism and Reactivity of Rh-Catalyzed Intermolecular [5+1] Cycloaddition of 3-Acyloxy-1,4-Enyne (ACE) and CO: A Computational Study.Chin Chem Lett. 2015 Jun;26(6):730-734. doi: 10.1016/j.cclet.2015.03.016. Epub 2015 Mar 27. Chin Chem Lett. 2015. PMID: 27152064 Free PMC article.
-
Rhodium-Catalyzed Stereoselective Intramolecular [5 + 2] Cycloaddition of 3-Acyloxy 1,4-Enyne and Alkene.Org Lett. 2015 Oct 16;17(20):5128-31. doi: 10.1021/acs.orglett.5b02665. Epub 2015 Oct 6. Org Lett. 2015. PMID: 26440751 Free PMC article.
-
Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions.Chem Rev. 2021 Jan 13;121(1):110-139. doi: 10.1021/acs.chemrev.0c00160. Epub 2020 Aug 5. Chem Rev. 2021. PMID: 32786421 Free PMC article.
References
-
-
For selected reviews on transition metal-catalyzed cycloadditions, see: Lautens M, Klute W, Tam W. Chem. Rev. 1996;96:49. Fruhauf HW. Chem. Rev. 1997;97:523. Aubert C, Buisine O, Malacria M. Chem. Rev. 2002;102:813. Evans PA. Modern Rhodium-Catalyzed Organic Reactions. Weinheim: Wiley-VCH; 2005. Michelet V, Toullec PY, Genet JP. Angew. Chem. Int. Ed. 2008;47:4268. Yu Z, Wang Y, Wang Y. Chem. Asian J. 2010;5:1072. Inglesby PA, Evans PA. Chem. Soc. Rev. 2010;39:2791.
-
-
-
For selected reviews on Diels-Alder cycloaddition, see: Danishefsky S. Acc. Chem. Res. 1981;14:400. Winkler JD. Chem. Rev. 1996;96:167. Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem. Int. Ed. 2002;41:1668. Takao K, Munakata R, Tadano K. Chem. Rev. 2005;105:4779.
-
-
-
For our communication of preliminary results on 1,3-acyloxy migration [5+1] cycloaddition with CO, see: Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew. Chem. Int. Ed. 2011;50:1346.
-
-
-
For comprehensive reviews on transition metal-mediated reactions involving cyclopropanes and cyclopropenes, see: Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. Zhu Z-B, Wei Y, Shi M. Chem. Soc. Rev. 2011;40:5534.
-
-
- Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew. Chem. Int. Ed. 2008;47:8933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

