Bone marrow-derived fibroblast growth factor-2 induces glial cell proliferation in the regenerating peripheral nervous system
- PMID: 22793996
- PMCID: PMC3503565
- DOI: 10.1186/1750-1326-7-34
Bone marrow-derived fibroblast growth factor-2 induces glial cell proliferation in the regenerating peripheral nervous system
Abstract
Background: Among the essential biological roles of bone marrow-derived cells, secretion of many soluble factors is included and these small molecules can act upon specific receptors present in many tissues including the nervous system. Some of the released molecules can induce proliferation of Schwann cells (SC), satellite cells and lumbar spinal cord astrocytes during early steps of regeneration in a rat model of sciatic nerve transection. These are the major glial cell types that support neuronal survival and axonal growth following peripheral nerve injury. Fibroblast growth factor-2 (FGF-2) is the main mitogenic factor for SCs and is released in large amounts by bone marrow-derived cells, as well as by growing axons and endoneurial fibroblasts during development and regeneration of the peripheral nervous system (PNS).
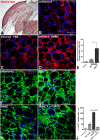
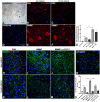
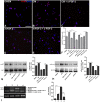
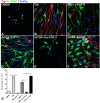
Results: Here we show that bone marrow-derived cell treatment induce an increase in the expression of FGF-2 in the sciatic nerve, dorsal root ganglia and the dorsolateral (DL) region of the lumbar spinal cord (LSC) in a model of sciatic nerve transection and connection into a hollow tube. SCs in culture in the presence of bone marrow derived conditioned media (CM) resulted in increased proliferation and migration. This effect was reduced when FGF-2 was neutralized by pretreating BMMC or CM with a specific antibody. The increased expression of FGF-2 was validated by RT-PCR and immunocytochemistry in co-cultures of bone marrow derived cells with sciatic nerve explants and regenerating nerve tissue respectivelly.
Conclusion: We conclude that FGF-2 secreted by BMMC strongly increases early glial proliferation, which can potentially improve PNS regeneration.
Figures
Similar articles
-
S100beta and fibroblast growth factor-2 are present in cultured Schwann cells and may exert paracrine actions on the peripheral nerve injury.Acta Cir Bras. 2008 Nov-Dec;23(6):555-60. doi: 10.1590/s0102-86502008000600014. Acta Cir Bras. 2008. PMID: 19030756
-
Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regeneration following sciatic nerve injury.Glia. 2014 Oct;62(10):1736-46. doi: 10.1002/glia.22712. Epub 2014 Jul 2. Glia. 2014. PMID: 24989458
-
Cellular analysis of S100Beta and fibroblast growth factor-2 in the dorsal root ganglia and sciatic nerve of rodents. focus on paracrine actions of activated satellite cells after axotomy.Int J Neurosci. 2007 Oct;117(10):1481-503. doi: 10.1080/15569520701502716. Int J Neurosci. 2007. PMID: 17729158
-
Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration--lessons from in vivo studies in mice and rats.Brain Res Rev. 2006 Aug;51(2):293-9. doi: 10.1016/j.brainresrev.2005.12.001. Epub 2006 Jan 23. Brain Res Rev. 2006. PMID: 16430964 Review.
-
Central and peripheral nerve regeneration by transplantation of Schwann cells and transdifferentiated bone marrow stromal cells.Anat Sci Int. 2002 Mar;77(1):12-25. doi: 10.1046/j.0022-7722.2002.00012.x. Anat Sci Int. 2002. PMID: 12418080 Review.
Cited by
-
EGFP transgene: a useful tool to track transplanted bone marrow mononuclear cell contribution to peripheral remyelination.Transgenic Res. 2018 Apr;27(2):135-153. doi: 10.1007/s11248-018-0062-5. Epub 2018 Feb 16. Transgenic Res. 2018. PMID: 29453733
-
Dual Contribution of Mesenchymal Stem Cells Employed for Tissue Engineering of Peripheral Nerves: Trophic Activity and Differentiation into Connective-Tissue Cells.Stem Cell Rev Rep. 2018 Apr;14(2):200-212. doi: 10.1007/s12015-017-9786-5. Stem Cell Rev Rep. 2018. PMID: 29214379
-
All the PNS is a Stage: Transplanted Bone Marrow Cells Play an Immunomodulatory Role in Peripheral Nerve Regeneration.ASN Neuro. 2023 Jan-Dec;15:17590914231167281. doi: 10.1177/17590914231167281. ASN Neuro. 2023. PMID: 37654230 Free PMC article.
-
Injured Nerve Regeneration using Cell-Based Therapies: Current Challenges.Acta Naturae. 2015 Jul-Sep;7(3):38-47. Acta Naturae. 2015. PMID: 26483958 Free PMC article.
-
Progression in translational research on spinal cord injury based on microenvironment imbalance.Bone Res. 2022 Apr 8;10(1):35. doi: 10.1038/s41413-022-00199-9. Bone Res. 2022. PMID: 35396505 Free PMC article. Review.
References
-
- Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RM, Mello-Silva JP, Cabral-da-Silva MC, de Mello FG, de Melo Reis RA, Mendez-Otero R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159:540–549. doi: 10.1016/j.neuroscience.2008.12.059. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

