Molecular characterization of multivalent bioconjugates by size-exclusion chromatography with multiangle laser light scattering
- PMID: 22794081
- PMCID: PMC3900495
- DOI: 10.1021/bc3000595
Molecular characterization of multivalent bioconjugates by size-exclusion chromatography with multiangle laser light scattering
Abstract
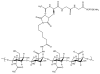
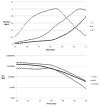
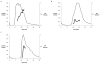
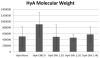
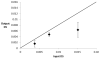
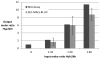
The degree of substitution and valency of bioconjugate reaction products are often poorly judged or require multiple time- and product-consuming chemical characterization methods. These aspects become critical when analyzing and optimizing the potency of costly polyvalent bioactive conjugates. In this study, size-exclusion chromatography with multiangle laser light scattering was paired with refractive index detection and ultraviolet spectroscopy (SEC-MALS-RI-UV) to characterize the reaction efficiency, degree of substitution, and valency of the products of conjugation of either peptides or proteins to a biopolymer scaffold, i.e., hyaluronic acid (HyA). Molecular characterization was more complete compared to estimates from a protein quantification assay, and exploitation of this method led to more accurate deduction of the molecular structures of polymer bioconjugates. Information obtained using this technique can improve macromolecular engineering design principles and help to better understand multivalent macromolecular interactions in biological systems.
Figures


References
-
- Elbert D, Hubbell J. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules. 2001;2:430–441. - PubMed
-
- Wall ST, Saha K, Ashton RS, Kam KR, Schaffer DV, Healy KE. Multivalency of Sonic hedgehog conjugated to linear polymer chains modulates protein potency. Bioconjug Chem. 2008;19:806–12. - PubMed
-
- Drumheller P, Elbert D, Hubbell J. Multifunctional poly (ethylene glycol) semi-interpenetrating polymer networks as highly selective adhesive substrates for bioadhesive peptide grafting. Biotechnology and bioengineering. 1994;43:772–780. - PubMed
-
- Stile RA, Healy KE. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules. 2001;2:185–94. - PubMed
-
- Ho M, Wang D, Hsieh H, Liu H, Hsien T, Lai J, Hou L. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials. 2005;26:3197–3206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

