Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection
- PMID: 22794198
- PMCID: PMC3557437
- DOI: 10.1089/ars.2012.4795
Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection
Abstract
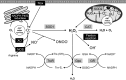
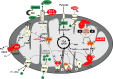
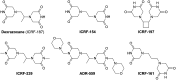
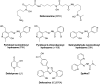
Significance: Anthracyclines (doxorubicin, daunorubicin, or epirubicin) rank among the most effective anticancer drugs, but their clinical usefulness is hampered by the risk of cardiotoxicity. The most feared are the chronic forms of cardiotoxicity, characterized by irreversible cardiac damage and congestive heart failure. Although the pathogenesis of anthracycline cardiotoxicity seems to be complex, the pivotal role has been traditionally attributed to the iron-mediated formation of reactive oxygen species (ROS). In clinics, the bisdioxopiperazine agent dexrazoxane (ICRF-187) reduces the risk of anthracycline cardiotoxicity without a significant effect on response to chemotherapy. The prevailing concept describes dexrazoxane as a prodrug undergoing bioactivation to an iron-chelating agent ADR-925, which may inhibit anthracycline-induced ROS formation and oxidative damage to cardiomyocytes.
Recent advances: A considerable body of evidence points to mitochondria as the key targets for anthracycline cardiotoxicity, and therefore it could be also crucial for effective cardioprotection. Numerous antioxidants and several iron chelators have been tested in vitro and in vivo with variable outcomes. None of these compounds have matched or even surpassed the effectiveness of dexrazoxane in chronic anthracycline cardiotoxicity settings, despite being stronger chelators and/or antioxidants.
Critical issues: The interpretation of many findings is complicated by the heterogeneity of experimental models and frequent employment of acute high-dose treatments with limited translatability to clinical practice.
Future directions: Dexrazoxane may be the key to the enigma of anthracycline cardiotoxicity, and therefore it warrants further investigation, including the search for alternative/complementary modes of cardioprotective action beyond simple iron chelation.
Figures





References
-
- Adachi K. Fujiura Y. Mayumi F. Nozuhara A. Sugiu Y. Sakanashi T. Hidaka T. Toshima H. A deletion of mitochondrial DNA in murine doxorubicin-induced cardiotoxicity. Biochem Biophys Res Commun. 1993;195:945–951. - PubMed
-
- Adams MJ. Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–606. - PubMed
-
- al-Harbi MM. al-Gharably NM. al-Shabanah OA. al-Bekairi AM. Osman AM. Tawfik HN. Prevention of doxorubicin-induced myocardial and haematological toxicities in rats by the iron chelator desferrioxamine. Cancer Chemother Pharmacol. 1992;31:200–204. - PubMed
-
- Al-Rousan RM. Paturi S. Laurino JP. Kakarla SK. Gutta AK. Walker EM. Blough ER. Deferasirox removes cardiac iron and attenuates oxidative stress in the iron-overloaded gerbil. Am J Hematol. 2009;84:565–570. - PubMed
-
- Alderton P. Gross J. Green MD. Role of (+-)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) in modulating free radical scavenging enzymes in doxorubicin-induced cardiomyopathy. Cancer Res. 1990;50:5136–5142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

