Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin
- PMID: 22794249
- PMCID: PMC3447992
- DOI: 10.1021/bi3006835
Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin
Abstract
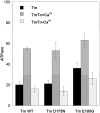
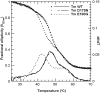
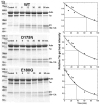
Cardiac α-tropomyosin (Tm) single-site mutations D175N and E180G cause familial hypertrophic cardiomyopathy (FHC). Previous studies have shown that these mutations increase both Ca(2+) sensitivity and residual contractile activity at low Ca(2+) concentrations, which causes incomplete relaxation during diastole resulting in hypertrophy and sarcomeric disarray. However, the molecular basis for the cause and the difference in the severity of the manifested phenotypes of disease are not known. In this work we have (1) used ATPase studies using reconstituted thin filaments in solution to show that these FHC mutants result in an increase in Ca(2+) sensitivity and an increased residual level of ATPase, (2) shown that both FHC mutants increase the rate of cleavage at R133, ~45 residues N-terminal to the mutations, when free and bound to actin, (3) shown that for Tm-E180G, the increase in the rate of cleavage is greater than that for D175N, and (4) shown that for E180G, cleavage also occurs at a new site 53 residues C-terminal to E180G, in parallel with cleavage at R133. The long-range decreases in dynamic stability due to these two single-site mutations suggest increases in flexibility that may weaken the ability of Tm to inhibit activity at low Ca(2+) concentrations for D175N and to a greater degree for E180G, which may contribute to differences in the severity of FHC.
Figures


References
-
- Watkins H, Seidman JG, Seidman CE. Familial hypertrophic cardiomyopathy: a genetic model of cardiac hypertrophy. Hum Mol Genet. 1995;4 Spec No:1721–1727. - PubMed
-
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. - PubMed
-
- Redwood CS, Moolman-Smook JC, Watkins H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc Res. 1999;44:20–36. - PubMed
-
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy. Cell. 1994;77:701–712. - PubMed
-
- Nakajima-Taniguchi C, Matsui H, Nagata S, Kishimoto T, Yamauchi-Takihara K. Novel missense mutation in alpha-tropomyosin gene found in Japanese patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol. 1995;27:2053–2058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

