Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation
- PMID: 22794264
- PMCID: PMC3422364
- DOI: 10.1016/j.neuron.2012.04.033
Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation
Erratum in
- Neuron. 2012 Sep 6;75(5):930
Abstract
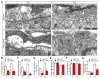
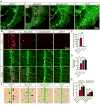
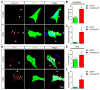
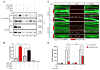
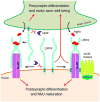
Neuromuscular junction (NMJ) formation requires precise interaction between motoneurons and muscle fibers. LRP4 is a receptor of agrin that is thought to act in cis to stimulate MuSK in muscle fibers for postsynaptic differentiation. Here we dissected the roles of LRP4 in muscle fibers and motoneurons in NMJ formation by cell-specific mutation. Studies of muscle-specific mutants suggest that LRP4 is involved in deciding where to form AChR clusters in muscle fibers, postsynaptic differentiation, and axon terminal development. LRP4 in HEK293 cells increased synapsin or SV2 puncta in contacting axons of cocultured neurons, suggesting a synaptogenic function. Analysis of LRP4 muscle and motoneuron double mutants and mechanistic studies suggest that NMJ formation may also be regulated by LRP4 in motoneurons, which could serve as agrin's receptor in trans to induce AChR clusters. These observations uncovered distinct roles of LRP4 in motoneurons and muscles in NMJ development.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

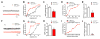

References
-
- Apel ED, Roberds SL, Campbell KP, Merlie JP. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995;15:115–126. - PubMed
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
-
- Bolis A, Coviello S, Bussini S, Dina G, Pardini C, Previtali SC, Malaguti M, Morana P, Del Carro U, Feltri ML, et al. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J Neurosci. 2005;25:8567–8577. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

