Associative fear learning enhances sparse network coding in primary sensory cortex
- PMID: 22794266
- PMCID: PMC3398404
- DOI: 10.1016/j.neuron.2012.04.035
Associative fear learning enhances sparse network coding in primary sensory cortex
Abstract
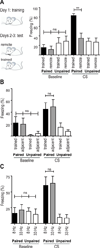
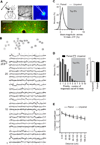
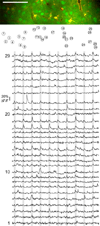
Several models of associative learning predict that stimulus processing changes during association formation. How associative learning reconfigures neural circuits in primary sensory cortex to "learn" associative attributes of a stimulus remains unknown. Using 2-photon in vivo calcium imaging to measure responses of networks of neurons in primary somatosensory cortex, we discovered that associative fear learning, in which whisker stimulation is paired with foot shock, enhances sparse population coding and robustness of the conditional stimulus, yet decreases total network activity. Fewer cortical neurons responded to stimulation of the trained whisker than in controls, yet their response strength was enhanced. These responses were not observed in mice exposed to a nonassociative learning procedure. Our results define how the cortical representation of a sensory stimulus is shaped by associative fear learning. These changes are proposed to enhance efficient sensory processing after associative learning.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

