A class of allosteric caspase inhibitors identified by high-throughput screening
- PMID: 22795132
- PMCID: PMC3428514
- DOI: 10.1016/j.molcel.2012.06.007
A class of allosteric caspase inhibitors identified by high-throughput screening
Abstract
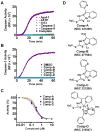
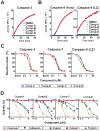
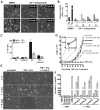
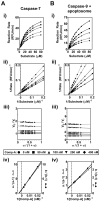
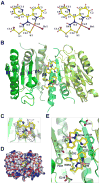
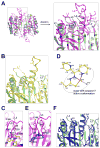
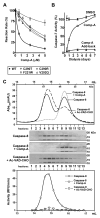
Caspase inhibition is a promising approach for treating multiple diseases. Using a reconstituted assay and high-throughput screening, we identified a group of nonpeptide caspase inhibitors. These inhibitors share common chemical scaffolds, suggesting the same mechanism of action. They can inhibit apoptosis in various cell types induced by multiple stimuli; they can also inhibit caspase-1-mediated interleukin generation in macrophages, indicating potential anti-inflammatory application. While these compounds inhibit all the tested caspases, kinetic analysis indicates they do not compete for the catalytic sites of the enzymes. The cocrystal structure of one of these compounds with caspase-7 reveals that it binds to the dimerization interface of the caspase, another common structural element shared by all active caspases. Consistently, biochemical analysis demonstrates that the compound abates caspase-8 dimerization. Based on these kinetic, biochemical, and structural analyses, we suggest that these compounds are allosteric caspase inhibitors that function through binding to the dimerization interface of caspases.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
A multipronged approach for compiling a global map of allosteric regulation in the apoptotic caspases.Methods Enzymol. 2014;544:215-49. doi: 10.1016/B978-0-12-417158-9.00009-1. Methods Enzymol. 2014. PMID: 24974292 Free PMC article.
-
Inhibition of caspase-9 by stabilized peptides targeting the dimerization interface.Biopolymers. 2012;98(5):451-65. doi: 10.1002/bip.22080. Biopolymers. 2012. PMID: 23203690 Free PMC article.
-
Identification of FDA-approved drugs as novel allosteric inhibitors of human executioner caspases.Proteins. 2018 Nov;86(11):1202-1210. doi: 10.1002/prot.25601. Epub 2018 Oct 5. Proteins. 2018. PMID: 30194780
-
Small Molecule Active Site Directed Tools for Studying Human Caspases.Chem Rev. 2015 Nov 25;115(22):12546-629. doi: 10.1021/acs.chemrev.5b00434. Epub 2015 Nov 9. Chem Rev. 2015. PMID: 26551511 Free PMC article. Review.
-
Modulating caspase activity: beyond the active site.Curr Opin Struct Biol. 2013 Dec;23(6):812-9. doi: 10.1016/j.sbi.2013.10.002. Epub 2013 Nov 8. Curr Opin Struct Biol. 2013. PMID: 24215810 Review.
Cited by
-
Microfluidic deep mutational scanning of the human executioner caspases reveals differences in structure and regulation.Cell Death Discov. 2022 Jan 10;8(1):7. doi: 10.1038/s41420-021-00799-0. Cell Death Discov. 2022. PMID: 35013287 Free PMC article.
-
MEF-AlloSite: an accurate and robust Multimodel Ensemble Feature selection for the Allosteric Site identification model.J Cheminform. 2024 Oct 23;16(1):116. doi: 10.1186/s13321-024-00882-5. J Cheminform. 2024. PMID: 39444016 Free PMC article.
-
The impact of cycleanine in cancer research: a computational study.J Mol Model. 2022 Oct 4;28(11):340. doi: 10.1007/s00894-022-05326-1. J Mol Model. 2022. PMID: 36194315
-
Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part II: Future compounds against influenza virus.J Prev Med Hyg. 2014 Dec;55(4):109-29. J Prev Med Hyg. 2014. PMID: 26137785 Free PMC article. Review.
-
Mechanisms of Gasdermin Recognition by Proteases.J Mol Biol. 2022 Feb 28;434(4):167274. doi: 10.1016/j.jmb.2021.167274. Epub 2021 Sep 29. J Mol Biol. 2022. PMID: 34599940 Free PMC article. Review.
References
-
- Acehan D, Jiang X, Morgan D, Heuzer J, Wang X, Akey C. Three-Dimensional Structure of the Apoptosome Implications for Assembly, Procaspase-9 Binding, and Activation. Molecular Cell. 2002;9:423–432. - PubMed
-
- Agniswamy J, Fang B, Weber IT. Conformational similarity in the activation of caspase-3 and -7 revealed by the unliganded and inhibited structures of caspase-7. Apoptosis. 2009;14:1135–1144. - PubMed
-
- Baici A. The specific velocity plot. A graphical method for determining inhibition parameters for both linear and hyperbolic enzyme inhibitors. Eur J Biochem. 1981;119:9–14. - PubMed
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

