Metabolic correction of congenital erythropoietic porphyria with iPSCs free of reprogramming factors
- PMID: 22795135
- PMCID: PMC3397263
- DOI: 10.1016/j.ajhg.2012.05.026
Metabolic correction of congenital erythropoietic porphyria with iPSCs free of reprogramming factors
Abstract
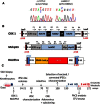
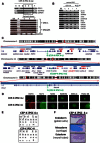
Congenital erythropoietic porphyria (CEP) is due to a deficiency in the enzymatic activity of uroporphyrinogen III synthase (UROS); such a deficiency leads to porphyrin accumulation and results in skin lesions and hemolytic anemia. CEP is a candidate for retrolentivirus-mediated gene therapy, but recent reports of insertional leukemogenesis underscore the need for safer methods. The discovery of induced pluripotent stem cells (iPSCs) has opened up new horizons in gene therapy because it might overcome the difficulty of obtaining sufficient amounts of autologous hematopoietic stem cells for transplantation and the risk of genotoxicity. In this study, we isolated keratinocytes from a CEP-affected individual and generated iPSCs with two excisable lentiviral vectors. Gene correction of CEP-derived iPSCs was obtained by lentiviral transduction of a therapeutic vector containing UROS cDNA under the control of an erythroid-specific promoter shielded by insulators. One iPSC clone, free of reprogramming genes, was obtained with a single proviral integration of the therapeutic vector in a genomic safe region. Metabolic correction of erythroblasts derived from iPSC clones was demonstrated by the disappearance of fluorocytes. This study reports the feasibility of porphyria gene therapy with the use of iPSCs.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Anderson K.E., Sassa S., Bishop D.F., Desnick R.J. Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. In: Scriver R., Beaudet A.L., Sly W.S., Valle E., editors. The Metabolic and Molecular Bases of Inherited Disease. C McGraw-Hill; New York: 2001. pp. 2961–3062.
-
- Richard E., Robert-Richard E., Ged C., Moreau-Gaudry F., de Verneuil H. Erythropoietic porphyrias: animal models and update in gene-based therapies. Curr. Gene Ther. 2008;8:176–186. - PubMed
-
- Shaw P.H., Mancini A.J., McConnell J.P., Brown D., Kletzel M. Treatment of congenital erythropoietic porphyria in children by allogeneic stem cell transplantation: A case report and review of the literature. Bone Marrow Transplant. 2001;27:101–105. - PubMed
-
- Kauffman L., Evans D.I.K., Stevens R.F., Weinkove C. Bone-marrow transplantation for congenital erythropoietic porphyria. Lancet. 1991;337:1510–1511. - PubMed
-
- Lagarde C., Hamel-Teillac D., De Prost Y., Blanche S., Thomas C., Fischer A., Nordmann Y., Ged C., De Verneuil H. [Allogeneic bone marrow transplantation in congenital erythropoietic porphyria. Gunther's disease] Ann. Dermatol. Venereol. 1998;125:114–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

