Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in mice with cashew allergy
- PMID: 22795369
- PMCID: PMC3810066
- DOI: 10.1016/j.jaci.2012.05.044
Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in mice with cashew allergy
Abstract
Background: IgE-mediated allergic reactions to cashews and other nuts can trigger life-threatening anaphylaxis. Proactive therapies to decrease reaction severity do not exist.
Objectives: We aimed to determine the efficacy of pepsin-digested cashew proteins used as immunotherapy in a murine model of cashew allergy.
Methods: Mice were sensitized to cashew and then underwent challenges with digested or native cashew allergens to assess the allergenicity of the protein preparations. Using native or pepsinized cashew proteins, mice underwent oral or intraperitoneal sensitization protocols to determine the immunogenic properties of the protein preparations. Finally, cashew-sensitized mice underwent an immunotherapy protocol with native or pepsinized cashew proteins and subsequent provocation challenges.
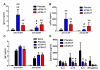
Results: Pepsinized cashew proteins elicited weaker allergic reactions than native cashew proteins but importantly retained the ability to stimulate cellular proliferation and cytokine production. Mice sensitized with pepsinized proteins reacted on challenge with native allergens, demonstrating that pepsinized allergens retain immunogenicity in vivo. Immunotherapy with pepsinized cashew allergens significantly decreased allergic symptoms and body temperature decrease relative to placebo after challenge with native and pepsinized proteins. Immunologic changes were comparable after immunotherapy with native or pepsinized allergens: T(H)2-type cytokine secretion from splenocytes was decreased, whereas specific IgG(1) and IgG(2a) levels were increased.
Conclusions: Pepsinized cashew proteins are effective in treating cashew allergy in mice and appear to work through the same mechanisms as native protein immunotherapy.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures

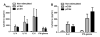

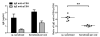

Similar articles
-
Development of an adjuvant-free cashew nut allergy mouse model.Int Arch Allergy Immunol. 2009;149(4):299-304. doi: 10.1159/000205575. Epub 2009 Mar 17. Int Arch Allergy Immunol. 2009. PMID: 19295233
-
Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation.Clin Rev Allergy Immunol. 2019 Aug;57(1):1-22. doi: 10.1007/s12016-016-8580-5. Clin Rev Allergy Immunol. 2019. PMID: 27585580 Review.
-
In vivo and T cell cross-reactivity between walnut, cashew and peanut.Int Arch Allergy Immunol. 2009;148(2):109-17. doi: 10.1159/000155741. Epub 2008 Sep 19. Int Arch Allergy Immunol. 2009. PMID: 18802355 Free PMC article.
-
Poly(anhydride) nanoparticles containing cashew nut proteins can induce a strong Th1 and Treg immune response after oral administration.Eur J Pharm Biopharm. 2018 Jun;127:51-60. doi: 10.1016/j.ejpb.2018.02.011. Epub 2018 Feb 8. Eur J Pharm Biopharm. 2018. PMID: 29428795
-
Production and analysis of recombinant tree nut allergens.Methods. 2014 Mar 1;66(1):34-43. doi: 10.1016/j.ymeth.2013.07.033. Epub 2013 Jul 31. Methods. 2014. PMID: 23911839 Review.
Cited by
-
A Specific Mixture of Fructo-Oligosaccharides and Bifidobacterium breve M-16V Facilitates Partial Non-Responsiveness to Whey Protein in Mice Orally Exposed to β-Lactoglobulin-Derived Peptides.Front Immunol. 2017 Jan 12;7:673. doi: 10.3389/fimmu.2016.00673. eCollection 2016. Front Immunol. 2017. PMID: 28127297 Free PMC article.
-
Could This Be IT? Epicutaneous, Sublingual, and Subcutaneous Immunotherapy for the Treatment of Food Allergies.Curr Allergy Asthma Rep. 2019 Nov 25;19(11):53. doi: 10.1007/s11882-019-0885-z. Curr Allergy Asthma Rep. 2019. PMID: 31768649 Review.
-
Preparation and in vitro bioactive evaluation of cashew-nut proteins hydrolysate as a potential source of anti-allergy peptides.J Food Sci Technol. 2021 Oct;58(10):3780-3789. doi: 10.1007/s13197-020-04838-z. Epub 2020 Oct 19. J Food Sci Technol. 2021. PMID: 34471301 Free PMC article.
-
Microbiological, Physicochemical, and Immunological Analysis of a Commercial Cashew Nut-Based Yogurt.Int J Mol Sci. 2020 Nov 4;21(21):8267. doi: 10.3390/ijms21218267. Int J Mol Sci. 2020. PMID: 33158240 Free PMC article.
-
Acid suppression therapy and allergic reactions.Allergo J Int. 2015 Dec;24(8):303-311. doi: 10.1007/s40629-015-0085-x. Allergo J Int. 2015. PMID: 28603686 Free PMC article.
References
-
- Clark S, Espinola JA, Rudders SA, Banerji A, Camargo CA., Jr. Frequency of US emergency department visits for food-related acute allergic reactions. J Allergy Clin Immunol. 2011;127:682–3. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–8. - PubMed
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. - PubMed
-
- Clark AT, Anagnostou K, Ewan PW. Cashew nut causes more severe reactions than peanut: case-matched comparison in 141 children. Allergy. 2007;62:913–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

