Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes
- PMID: 22795370
- PMCID: PMC3488163
- DOI: 10.1016/j.jaci.2012.05.036
Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes
Abstract
Background: IL-17 is an important cytokine signature of the TH differentiation pathway TH17. This T-cell subset is crucial in mediating autoimmune disease or antimicrobial immunity in animal models, but its presence and role in human disease remain to be completely characterized.
Objective: We set out to determine the frequency of TH17 cells in patients with cystic fibrosis (CF), a disease in which there is recurrent infection with known pathogens.
Methods: Explanted lungs from patients undergoing transplantation or organ donors (CF samples=18; non-CF, nonbronchiectatic samples=10) were collected. Hilar nodes and parenchymal lung tissue were processed and examined for TH17 signature by using immunofluorescence and quantitative real-time PCR. T cells were isolated and stimulated with antigens from Pseudomonas aeruginosa and Aspergillus species. Cytokine profiles and staining with flow cytometry were used to assess the reactivity of these cells to antigen stimulation.
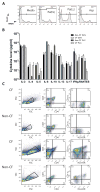
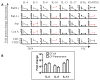
Results: We found a strong IL-17 phenotype in patients with CF compared with that seen in control subjects without CF. Within this tissue, we found pathogenic antigen-responsive CD4+IL-17+ cells. There were double-positive IL-17+IL-22+ cells [TH17(22)], and the IL-22+ population had a higher proportion of memory characteristics. Antigen-specific TH17 responses were stronger in the draining lymph nodes compared with those seen in matched parenchymal lungs.
Conclusion: Inducible proliferation of TH17(22) with memory cell characteristics is seen in the lungs of patients with CF. The function of these individual subpopulations will require further study regarding their development. T cells are likely not the exclusive producers of IL-17 and IL-22, and this will require further characterization.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures

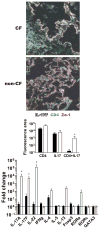



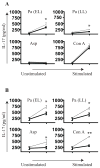
 ) and compared to no neutralization (—) with 20 μg/ml of IgG. (B) IL-13 and IFNγ were also neutralized (n = 3).. * p < 0.05, ** p < 0.001
) and compared to no neutralization (—) with 20 μg/ml of IgG. (B) IL-13 and IFNγ were also neutralized (n = 3).. * p < 0.05, ** p < 0.001References
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. - PubMed
-
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. - PubMed
-
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. - PubMed
-
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

