Environmental enrichment restores neurogenesis and rapid acquisition in aged rats
- PMID: 22795793
- PMCID: PMC3480541
- DOI: 10.1016/j.neurobiolaging.2012.05.023
Environmental enrichment restores neurogenesis and rapid acquisition in aged rats
Abstract
Strategies combatting cognitive decline among the growing aging population are vital. We tested whether environmental enrichment could reverse age-impaired rapid spatial search strategy acquisition concomitantly with hippocampal neurogenesis in rats. Young (5-8 months) and aged (20-22 months) male Fischer 344 rats were pair-housed and exposed to environmental enrichment (n = 7 young, 9 aged) or housed individually (n = 7 young, 7 aged) for 10 weeks. After 5 weeks, hidden platform trials (5 blocks of 3 trials; 15 m inter-block interval), a probe trial, and then visible platform trials (5 blocks of 3 trials; 15 m inter-block interval) commenced in the water maze. One week after testing, rats were given 5 daily intraperitoneal bromodeoxyuridine (50 mg/kg) injections and perfused 4 weeks later to quantify neurogenesis. Although young rats outperformed aged rats, aged enriched rats outperformed aged individually housed rats on all behavioral measures. Neurogenesis decreased with age but enrichment enhanced new cell survival, regardless of age. The novel correlation between new neuron number and behavioral measures obtained in a rapid water maze task among aged rats, suggests that environmental enrichment increases their ability to rapidly acquire and flexibly use spatial information along with neurogenesis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that all research reported in this manuscript was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

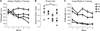




Similar articles
-
Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory.J Neurosci. 2011 Jul 6;31(27):9933-44. doi: 10.1523/JNEUROSCI.1062-11.2011. J Neurosci. 2011. PMID: 21734285 Free PMC article.
-
Cannabinoid agonist WIN-55,212-2 partially restores neurogenesis in the aged rat brain.Mol Psychiatry. 2009 Dec;14(12):1068-9. doi: 10.1038/mp.2009.62. Mol Psychiatry. 2009. PMID: 19920834 Free PMC article. No abstract available.
-
Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning.Neuroscience. 2005;135(2):395-402. doi: 10.1016/j.neuroscience.2005.06.065. Neuroscience. 2005. PMID: 16125851
-
Intracranial self-stimulation enhances neurogenesis in hippocampus of adult mice and rats.Neuroscience. 2009 Jan 23;158(2):402-11. doi: 10.1016/j.neuroscience.2008.10.048. Epub 2008 Nov 7. Neuroscience. 2009. PMID: 19041373
-
Environmentally induced long-term structural changes: cues for functional orientation and vulnerabilities.Neurotox Res. 2004;6(7-8):571-80. doi: 10.1007/BF03033453. Neurotox Res. 2004. PMID: 15639789 Review.
Cited by
-
Cellular and molecular signatures of motherhood in the adult and ageing rat brain.Open Biol. 2023 Nov;13(11):230217. doi: 10.1098/rsob.230217. Epub 2023 Nov 22. Open Biol. 2023. PMID: 37989220 Free PMC article.
-
Age, environment, object recognition and morphological diversity of GFAP-immunolabeled astrocytes.Behav Brain Funct. 2016 Oct 10;12(1):28. doi: 10.1186/s12993-016-0111-2. Behav Brain Funct. 2016. PMID: 27719674 Free PMC article.
-
Adult Hippocampal Neurogenesis: A Coming-of-Age Story.J Neurosci. 2018 Dec 5;38(49):10401-10410. doi: 10.1523/JNEUROSCI.2144-18.2018. Epub 2018 Oct 31. J Neurosci. 2018. PMID: 30381404 Free PMC article. Review.
-
Environmental Enrichment Rescues Visually-Mediated Behavior in Ten-m3 Knockout Mice During an Early Critical Period.Front Behav Neurosci. 2020 Feb 25;14:22. doi: 10.3389/fnbeh.2020.00022. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32158383 Free PMC article.
-
iPlasticity: Induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants.Psychiatry Clin Neurosci. 2018 Sep;72(9):633-653. doi: 10.1111/pcn.12683. Epub 2018 Jul 11. Psychiatry Clin Neurosci. 2018. PMID: 29802758 Free PMC article. Review.
References
-
- Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. - PubMed
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. - PubMed
-
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. - PubMed
-
- Camel JE, Withers GS, Greenough WT. Persistence of visual cortex dendritic alterations induced by postweaning exposure to a "superenriched" environment in rats. Behav Neurosci. 1986;100:810–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

