The osteogenic differentiation of human bone marrow MSCs on HUVEC-derived ECM and β-TCP scaffold
- PMID: 22795852
- PMCID: PMC3427692
- DOI: 10.1016/j.biomaterials.2012.06.061
The osteogenic differentiation of human bone marrow MSCs on HUVEC-derived ECM and β-TCP scaffold
Abstract
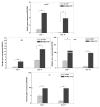
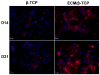
Extracellular matrix (ECM) serves a key role in cell migration, attachment, and cell development. Here we report that ECM derived from human umbilical vein endothelial cells (HUVEC) promoted osteogenic differentiation of human bone marrow mesenchymal stem cells (hMSC). We first produced an HUVEC-derived ECM on a three-dimensional (3D) beta-tricalcium phosphate (β-TCP) scaffold by HUVEC seeding, incubation, and decellularization. The HUVEC-derived ECM was then characterized by SEM, FTIR, XPS, and immunofluorescence staining. The effect of HUVEC-derived ECM-containing β-TCP scaffold on hMSC osteogenic differentiation was subsequently examined. SEM images indicate a dense matrix layer deposited on the surface of struts and pore walls. FTIR and XPS measurements show the presence of new functional groups (amide and hydroxyl groups) and elements (C and N) in the ECM/β-TCP scaffold when compared to the β-TCP scaffold alone. Immunofluorescence images indicate that high levels of fibronectin and collagen IV and low level of laminin were present on the scaffold. ECM-containing β-TCP scaffolds significantly increased alkaline phosphatase (ALP) specific activity and up-regulated expression of osteogenesis-related genes such as runx2, alkaline phosphatase, osteopontin and osteocalcin in hMSC, compared to β-TCP scaffolds alone. This increased effect was due to the activation of MAPK/ERK signaling pathway since disruption of this pathway using an ERK inhibitor PD98059 results in down-regulation of these osteogenic genes. Cell-derived ECM-containing calcium phosphate scaffolds is a promising osteogenic-promoting bone void filler in bone tissue regeneration.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures









References
-
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. - PubMed
-
- Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC. Use of extracellular matrix components for cell culture. Anal Biochem. 1987;166:1–13. - PubMed
-
- Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J biomech. 2010;43:55–62. - PubMed
-
- Flynn LE, Prestwich GD, Semple JL, Woodhouse KA. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29:1862–71. - PubMed
-
- Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006;12:319–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

