Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors
- PMID: 22796081
- PMCID: PMC3443632
- DOI: 10.1016/j.neuroscience.2012.07.006
Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors
Abstract
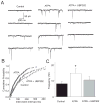
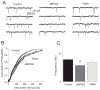
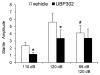
Kainate receptors containing the GluK1 subunit (GluK1Rs; previously known as GluR5 kainate receptors) are concentrated in certain brain regions, where they play a prominent role in the regulation of neuronal excitability, by modulating GABAergic and/or glutamatergic synaptic transmission. In the basolateral nucleus of the amygdala (BLA), which plays a central role in anxiety as well as in seizure generation, GluK1Rs modulate GABAergic inhibition via postsynaptic and presynaptic mechanisms. However, the role of these receptors in the regulation of glutamate release, and the net effect of their activation on the excitability of the BLA network are not well understood. Here, we show that in amygdala slices from 35- to 50-day-old rats, the GluK1 agonist (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA) (300 nM) increased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) and miniature EPSCs (mEPSCs) recorded from BLA principal neurons, and decreased the rate of failures of evoked EPSCs. The GluK1 antagonist (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl) pyrimidine-2,4-dione (UBP302) (25 or 30 μM) decreased the frequency of mEPSCs, reduced evoked field potentials, and increased the "paired-pulse ratio" of the field potential amplitudes. Taken together, these results suggest that GluK1Rs in the rat BLA are present on presynaptic terminals of principal neurons, where they mediate facilitation of glutamate release. In vivo bilateral microinjections of ATPA (250 pmol) into the rat BLA increased anxiety-like behavior in the open field test, while 2 nmol ATPA induced seizures. Similar intra-BLA injections of UBP302 (20 nmol) had anxiolytic effects in the open field and the acoustic startle response tests, without affecting pre-pulse inhibition. These results suggest that although GluK1Rs in the rat BLA facilitate both GABA and glutamate release, the facilitation of glutamate release prevails, and these receptors can have an anxiogenic and seizurogenic net function. Presynaptic facilitation of glutamate release may, in part, underlie the hyperexcitability-promoting effects of GluK1R activation in the rat BLA.
Published by Elsevier Ltd.
Figures




References
-
- Alt A, Weiss B, Ogden AM, Li X, Gleason SD, Calligaro DO, Bleakman D, Witkin JM. In vitro and in vivo studies in rats with LY293558 suggest AMPA/kainate receptor blockade as a novel potential mechanism for the therapeutic treatment of anxiety disorders. Psychopharmacology (Berl) 2006;185:240–247. - PubMed
-
- Alt A, Weiss B, Ornstein PL, Gleason SD, Bleakman D, Stratford RE, Jr, Witkin JM. Anxiolytic-like effects through a GLUK5 kainate receptor mechanism. Neuropharmacology. 2007;52:1482–1487. - PubMed
-
- Aroniadou-Anderjaska V, Qashu F, Maria Braga MF. Mechanisms Regulating GABAergic Inhibitory Transmission in the Basolateral Amygdala: Implications for Epilepsy and Anxiety Disorders. Amino Acids. 2007;32:305–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

