Ultrasound-guided percutaneous delivery of tissue-engineered endothelial cells to the adventitia of stented arteries controls the response to vascular injury in a porcine model
- PMID: 22796118
- PMCID: PMC3762489
- DOI: 10.1016/j.jvs.2012.03.002
Ultrasound-guided percutaneous delivery of tissue-engineered endothelial cells to the adventitia of stented arteries controls the response to vascular injury in a porcine model
Erratum in
- J Vasc Surg. 2012 Dec;56(6):1831
Abstract
Objective: High restenosis rates are a limitation of peripheral vascular interventions. Previous studies have shown that surgical implantation of a tissue-engineered endothelium onto the adventitia surface of injured vessels regulates vascular repair. In the present study, we developed a particulate formulation of tissue-engineered endothelium and a method to deliver the formulation perivascular to injured blood vessels using a percutaneous, minimally invasive technique.
Methods: Stainless steel stents were implanted in 18 balloon-injured femoral arteries of nine domestic swine, followed by ultrasound-guided percutaneous perivascular injection of gelatin particles containing cultured allogeneic porcine aortic endothelial cells (PAE). Controls received injections of empty particles (matrix) or no perivascular injection (sham) after stent deployment. Animals were sacrificed after 90 days.
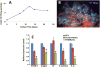
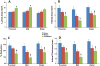
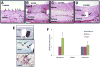
Results: Angiographic analysis revealed a significantly greater lumen diameter in the stented segments of arteries treated with PAE/matrix (4.72 ± 0.12 mm) compared with matrix (4.01 ± 0.20 mm) or sham (4.03 ± 0.16 mm) controls (P < .05). Similarly, histologic analysis revealed that PAE/matrix-treated arteries had the greatest lumen area (20.4 ± 0.7 mm(2); P < .05) compared with controls (16.1 ± 0.9 mm(2) and 17.1 ± 1.0 mm(2) for sham and matrix controls, respectively) and the smallest intimal area (3.3 ± 0.4 mm(2); P < .05) compared with controls (6.2 ± 0.5 mm(2) and 4.4 ± 0.5 mm(2) for sham and matrix controls, respectively). Overall, PAE-treated arteries had a 33% to 50% decrease in percent occlusion (P < .05) compared with controls. Histopathological analysis revealed fewer leukocytes present in the intima in the PAE/matrix group compared with control groups, suggesting that the biological effects were in part due to inhibition of the inflammatory phase of the vascular response to injury.
Conclusions: Minimally invasive, perivascular delivery of PAE/matrix to stented arteries was performed safely using ultrasound-guided percutaneous injections and significantly decreased stenosis. Application at the time of or subsequent to peripheral interventions may decrease clinical restenosis rates.
Copyright © 2012 Society for Vascular Surgery. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Author conflict of interest: Dr Nugent, Dr Ng, Mr White, and Mr Kanner are or were employees of and have shares in Pervasis Therapeutics. Elazer Edelman is on the board of directors and has shares in Pervasis Therapeutics.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Figures


Comment in
-
Keeping the channels open: allogeneic endothelial cells modulate restenosis of injured blood vessels.Regen Med. 2012 Nov;7(6):751-2. doi: 10.2217/rme.12.93. Regen Med. 2012. PMID: 23164076 No abstract available.
Similar articles
-
Delivery site of perivascular endothelial cell matrices determines control of stenosis in a porcine femoral stent model.J Vasc Interv Radiol. 2009 Dec;20(12):1617-24. doi: 10.1016/j.jvir.2009.08.020. Epub 2009 Oct 24. J Vasc Interv Radiol. 2009. PMID: 19854069 Free PMC article.
-
Effect of endovascular stent strut geometry on vascular injury, myointimal hyperplasia, and restenosis.J Vasc Surg. 2002 Jul;36(1):143-9. doi: 10.1067/mva.2002.122878. J Vasc Surg. 2002. PMID: 12096272
-
Stents covered by an autologous arterial graft in porcine coronary arteries: feasibility, vascular injury and effect on neointimal hyperplasia.Cardiovasc Res. 1999 Feb;41(2):433-42. doi: 10.1016/s0008-6363(98)00211-9. Cardiovasc Res. 1999. PMID: 10341842
-
Comparative pathology: radiation-induced coronary artery disease in man and animals.Semin Interv Cardiol. 1998 Sep-Dec;3(3-4):163-72. Semin Interv Cardiol. 1998. PMID: 10406688 Review.
-
The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation.Int J Radiat Oncol Biol Phys. 1996 Nov 1;36(4):789-96. doi: 10.1016/s0360-3016(96)00299-4. Int J Radiat Oncol Biol Phys. 1996. PMID: 8960504 Review.
Cited by
-
Finite element modeling of a novel self-expanding endovascular stent method in treatment of aortic aneurysms.Sci Rep. 2014 Jan 10;4:3630. doi: 10.1038/srep03630. Sci Rep. 2014. PMID: 24406869 Free PMC article.
-
A novel endothelial-derived anti-inflammatory activity significantly inhibits spontaneous choroidal neovascularisation in a mouse model.Vasc Cell. 2016 May 11;8:2. doi: 10.1186/s13221-016-0036-4. eCollection 2016. Vasc Cell. 2016. PMID: 27175278 Free PMC article.
-
The role of cystatin C in vascular remodeling of balloon-injured abdominal aorta of rabbits.Mol Biol Rep. 2014 Sep;41(9):6225-31. doi: 10.1007/s11033-014-3502-1. Epub 2014 Jul 1. Mol Biol Rep. 2014. Retraction in: Mol Biol Rep. 2015 Oct;42(10):1475. doi: 10.1007/s11033-015-3901-y. PMID: 24981928 Retracted.
-
Novel engineered, membrane-localized variants of vascular endothelial growth factor (VEGF) protect retinal ganglion cells: a proof-of-concept study.Cell Death Dis. 2018 Oct 3;9(10):1018. doi: 10.1038/s41419-018-1049-0. Cell Death Dis. 2018. PMID: 30282966 Free PMC article.
References
-
- Minar E, Pokrajac B, Maca T, Ahmadi R, Fellner C, Mittlböck M, et al. Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angioplasty: results of a prospective randomized study. Circulation. 2000;102:2694–9. - PubMed
-
- Grimm J, Müller-Hülsbeck S, Jahnke T, Hilbert C, Brossmann J, Heller M. Randomized study to compare PTA alone versus PTA with Palmaz stent placement for femoropopliteal lesions. J Vasc Interv Radiol. 2001;12:935–42. - PubMed
-
- Cejna M, Thurnher S, Illiasch H, Horvath W, Waldenberger P, Hornik K, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: A multicenter prospective randomized study. J Vasc Interv Radiol. 2001;12:23–31. - PubMed
-
- Tepe G. Drug-eluting stents for infrainguinal occlusive disease: progress and challenges. Semin Vasc Surg. 2006;19:102–8. - PubMed
-
- Machan L. Drug eluting stents in the infrainguinal circulation. Tech Vasc Interv Radiol. 2004;7:28–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

